Chemical, Biological, Radiological and Nuclear (CBRN) Injury Part 1: Initial Response to CBRN Agents
Joint Trauma System
Chemical, Biological, Radiological and Nuclear (CBRN) Injury Part I: Initial Response to CBRN Agents
INTRODUCTION
This Role 1 (including Point of Injury [POI]) through Role 3 Clinical Practice Guideline (CPG) is intended for use in conjunction with Tactical Combat Casualty Care (TCCC) Guidelines. This CPG is meant to provide medical professionals who encounter CBRN exposures with evidence-based guidance where it exists or consensus opinion when evidence is lacking. This CPG is divided into 4 sections:
- Response to CBRN agents (an approach to all CBRN casualties)
- Medical management of chemical agent exposure
- Medical management of radiation exposure and nuclear events
- Medical management of biologic agent exposure
While encountering conventional casualties on the battlefield remains a more likely scenario, vigilance is necessary for unusual scenarios that might represent the first clue that a CBRN incident occurred. Since environmental sampling for agent detection and rapid point-of-care testing for agent identification will not be routinely available in austere environments or most hospital settings, the initial suspicion of a CBRN attack will rest on clinical recognition of signs and symptoms of poisoning. This guideline is intended to provide an approach to casualty assessment and treatment from POI to established medical facilities.
Basic Principles of CBRN Casualty Management
Initial care of the CBRN casualty should be approached in the same manner as other casualties. Life threats require prompt recognition and intervention, and non-life-threatening sequelae can be addressed when clinically appropriate. Early recognition and categorization of CBRN-exposed patients is the foundation for further management, and is key not only for initiating patient treatment but also for preventing contamination of medical personnel, equipment, and facilities. Thorough and appropriate decontamination is a core skill that requires planning and practice. Attention to details such as preventing hypothermia in patients undergoing decontamination and clinical reassessment at each stage of the process will reduce unnecessary morbidity. Basic life saving measures such as airway management and resuscitation are fundamental concepts that must be mastered at the appropriate level for each practitioner in the CBRN care chain. Furthermore, easy access to reference materiel to guide advanced therapy should be a part of every provider’s armamentarium. These basic principles will be discussed in more detail in the sections that follow.
Critical Task List
The critical task list is applicable to both medical and non-medical personnel. All personnel should be trained on the concepts and principles identified in the critical task list in order to respond to CBRN casualties. The critical task list addresses core competencies that can be adjusted based on the medical skill level of the responder. These tasks should be trained according to service-specific publications and recognized standards of medical care.
- Recognize CBRN exposure that requires action to protect self and others
- Don personal protective equipment (PPE) to prevent exposure in self and assist others with PPE
- Egress from the threat
- Move upwind, uphill, upstream from threat
- Utilize time/distance/shielding for protection
- Recognize signs/symptoms of CBRN exposure that prompt immediate self-treatment or treatment of others (i.e. nerve agent exposure)
- Apply TCCC integrated with CBRN response TCCC + CBRN = (MARCHE)2
Massive hemorrhage/mask, airway/antidote, respiration/rapid spot decontamination, circulation/ countermeasure, hypothermia/head injury, extraction/evacuation
- Apply airway management skills in a CBRN setting (positioning, suction, ventilation to include manual and mechanical, placement of definitive airway)
- Perform Rapid Spot Decontamination
- Identify and establish Hot/Warm/Cold Zones
- Establish a dirty casualty collection point (CCP)
- Understand decontamination principles and casualty procedures for partial or complete removal of PPE, clothing, and equipment (casualty cut out)
- Understand cross contamination and take appropriate measures to prevent it
- Understand available technology that can aid in agent identification
Planning Considerations
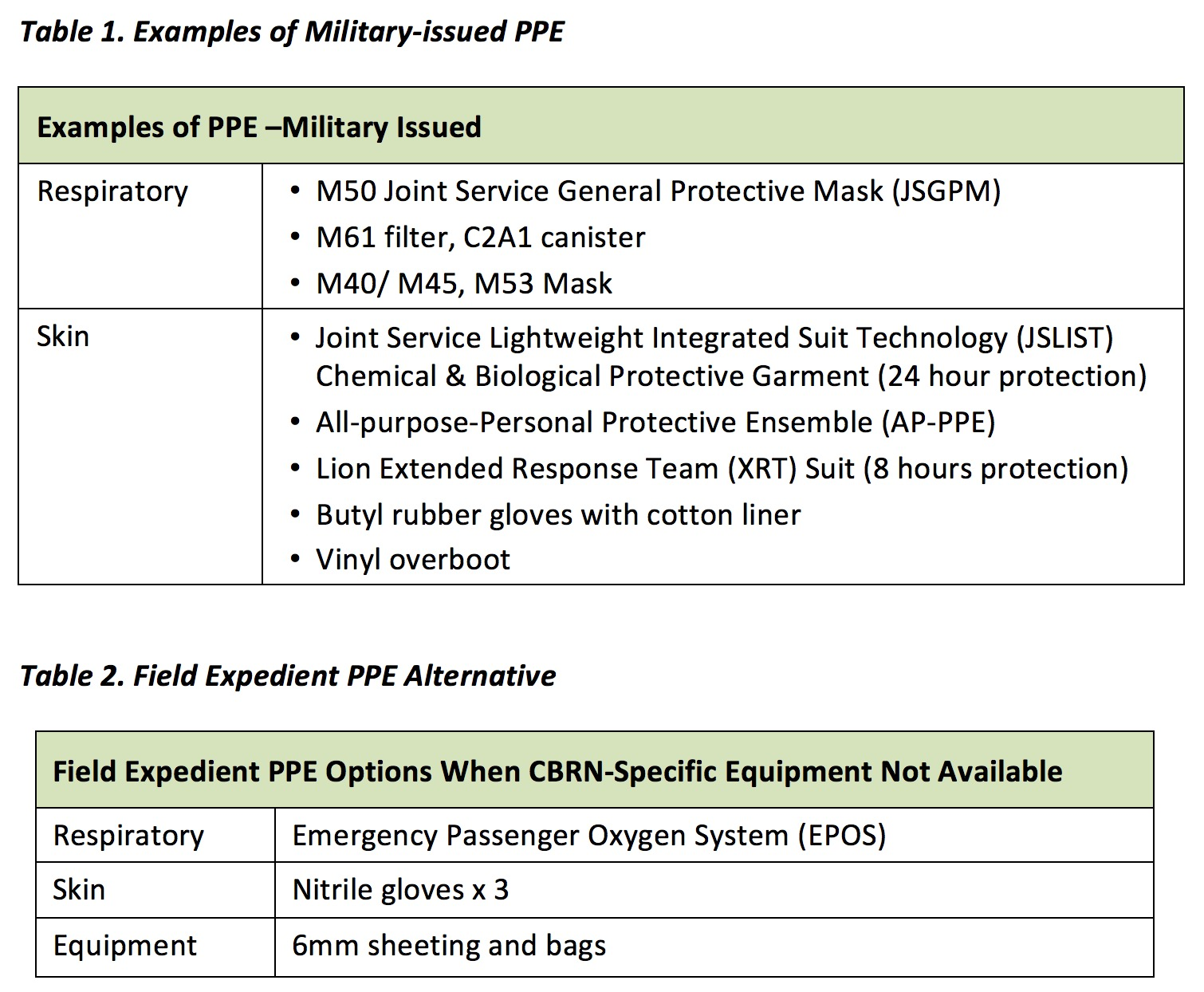
The response to CBRN exposure requires extensive planning based on the threat. In a high-threat environment, immediate availability of adequate PPE is essential. Personnel should also be aware of field expedient PPE options. (Table 1)
Evacuation Planning Considerations
Medical Regulating
1. Military Treatment Facility (MTF)
- DECON/Treatment Coordination. Ensure MTF is prepared to receive dirty casualties and determine the most appropriate location for DECON.
- Treatment Capabilities (Toxicology, Critical Care, Trauma Surgery). Determine whether the MTF has the services necessary to care for and sustain the CBRN casualty on site and/or establish telemedicine support.
- Capacity. The CBRN casualty is far more resource intensive than a typical trauma or critically ill casualty. Assess the MTF’s capacity and capability to treat CBRN casualties and identify potential alternate locations.
2. Evaluation. Integrate the medical regulating system into CBRN exercise scenarios.
Landing Zone/Casualty Exchange Points
1. Route coordination. Consider alternate routes, primary routes may be jammed or unavailable.
2. Environmental Considerations
- Wind
- Terrain / Slope
- Drainage
- Water sources
Evacuation Platforms
- Clean and Dirty. It is necessary to plan for both clean and dirty platforms for evacuation.
- Refuel. When planning evacuations, consider the time it takes for refueling in a MASCAL situation, as well as the distance from the objective to the DECON site and MTF. Also factor in any platform decontamination that may be necessary prior to arrival at the refueling site.
- Aircraft / Vehicle DECON (Spot, Wash Down, Detailed). Based on the available assets, determine the time it will take to conduct each type of decontamination.
- Preparation time (hasty vs. deliberate). Factor the time it takes to prepare the platform for a hasty or deliberate CBRN mission.
- Evaluation. Integrate evacuation and handoff training into CBRN training exercises.
Protective Measures (allow for time required to install when planning)
1. Barrier (disposable; plan for additional on-hand quantities)
- Preventive maintenance checks need to occur on a regular basis.
- Consider time required for deliberate vs. hasty set-up.
- 6mm tarp and chemical or 100-mph tape for improvised
2. PPE (Pilots / Drivers / Crew)
Casualty Care
Class VIII (medical supplies) and critical care support equipment:
1. Antidote and countermeasure resupply and additional stock (information on the CDC’s Strategic National Stockpile can be found at https://www.cdc.gov/phpr/stockpile
2. Airway support / ventilation - anticipate need for advanced airway management and ventilators
3. Casualty Monitoring
- Electronic vital signs monitoring
- ETCO2
- Hypothermia. Special emphasis must be placed on maintaining body temperature, as cut-out procedures will create greater exposure to environmental factors.
Decontamination
An extensive discussion of decontamination is outside the scope of this CPG, however basic decontamination principles are outlined below. (Figure 1)
1. Prevent further casualties by applying PPE before treating others.
2. Remove all clothing and equipment. Contain and dispose of contaminated material appropriately.
3. If radiologic exposure, cover wounds before further decontamination. Ensure gentle decontamination as radionuclide absorption may be increased if skin becomes erythematous.
4. Decontamination Solutions
a. Soap and water in copious amounts
- Physical removal and dilution of agents
- Does not destroy biological agents or neutralize radioactive particles
b. 0.5% Hypochlorite Solution
- Nine parts water to one part 5% bleach
- Wipe on the skin and rinse with fresh water
- Can be used in open wounds but do not use in chest or abdominal cavity (risk of adhesions), open brain or spinal cord wounds (unknown effects), or in the eyes (risk of corneal opacification)
c. Reactive Skin Decontamination Lotion (RSDL)
- Indicated for chemical agents and T-2 mycotoxin
- Packaged sponge contains reactive agent that penetrates skin
- Deactivates mustard (HD) and nerve agent
- Currently FDA-approved only for intact skin, not for wounds or eyes
- Best results occur when RSDL is applied immediately after exposure, gently scrubbed (agitated) on skin for two minutes, removed, and then reapplied (RSDL may be left on skin for up to 24 hours)
d. Surgical solutions for open chest and abdominal cavity wounds
e. Water, saline, or eye solutions are recommended for the eye
5. Standard infectious disease protective measures should be utilized for biological agents
6. Internal decontamination will be discussed in detail in the medical management of radiologic exposure and nuclear events
Identification of Agent
Agent identification is a critical step in determining casualty treatment. Recognize that intelligence reporting may be incomplete or inadequate. There are a variety of detectors that can aid in agent identification and providers should be familiar with the technology that is part of their organization.
Combine available intelligence, technology, and patient presentation to create a comprehensive picture for agent identification to help guide treatment. Mixed exposures are possible and correlating patient signs and symptoms with adjunctive agent identification techniques can help ensure the proper treatment is pursued.
** Casualty treatment should never be delayed pending confirmatory agent identification. Clinical assessment (using CRESS described below) is necessary to determine immediate therapy. Decisive efforts for agent identification can be done in parallel to timely medical treatment.**
Triage and Evacuation Priorities
Because of the differences in casualty presentation for chemical, biological, radiological, and nuclear event casualties, specific triage recommendations and evacuation priority considerations will be addressed in the portion of the CPG that is specific to the categories of CBRN exposures.
Do not forget that CBRN exposure is an enemy attack and security will be the first priority to be coordinated. Then, triage for a CBRN event follows established triage principles, including doing the most good for the most people. Because of the complexity of combined CBRN and trauma casualties, it may be beneficial to assign an experienced provider to triage. Triage occurs for decontamination, for medical treatment, and for evacuation. Although placing casualties into one of two groups for assignment to ambulatory vs. non-ambulatory decontamination lanes is a kind of sorting, true triage for decontamination involves a determination of who can tolerate delays in decontamination. Decontamination order may be based on extent of exposure and not on initial symptoms, as symptoms may be delayed. Those with more extensive exposures may benefit from rapid decontamination to decrease their risk for later symptoms, and thus reduce the future burden on medical resources. Once decontaminated, patients are triaged according to medical priority. Decontamination is a medical intervention (it minimizes or eliminates the conversion of an external dose to an internal dose), and any casualty with an area suspicious of having been exposed to a liquid chemical agent is automatically medically triaged as immediate until immediate (local, spot) decontamination has been accomplished. The casualty is then re-triaged. Except for vesicant casualties, those exposed only to vapor can tolerate delays in decontamination or may not even require thorough decontamination. It is important to frequently reassess patients. Because many CBRN agents cause delayed symptoms, a casualty initially determined to be minimal may become immediate. (Ramesh 2010) Remember that the process of decontamination can significantly change the casualty status. It is critically important to monitor for hypothermia, disruption of previously controlled bleeding, and other changes in patient status that would change triage priority.
Tactical Combat CBRN Casualty Care + CBRN
Assessment and management of CBRN casualties is complex because it involves not only life-threatening symptoms (some of them rapid onset) but also the threat of contamination of all first responders. An added source of complexity is the frequent mixing of CBRN and penetrating trauma injuries. (Figure 2) Working through this complexity involves a stepwise approach that first rules in or rules out CBRN injuries so that first responders can protect themselves if necessary before rendering aid. The next step integrates assessment and management of both CBRN and traumatic injuries, prioritizing the recognition and management of life-threats first.
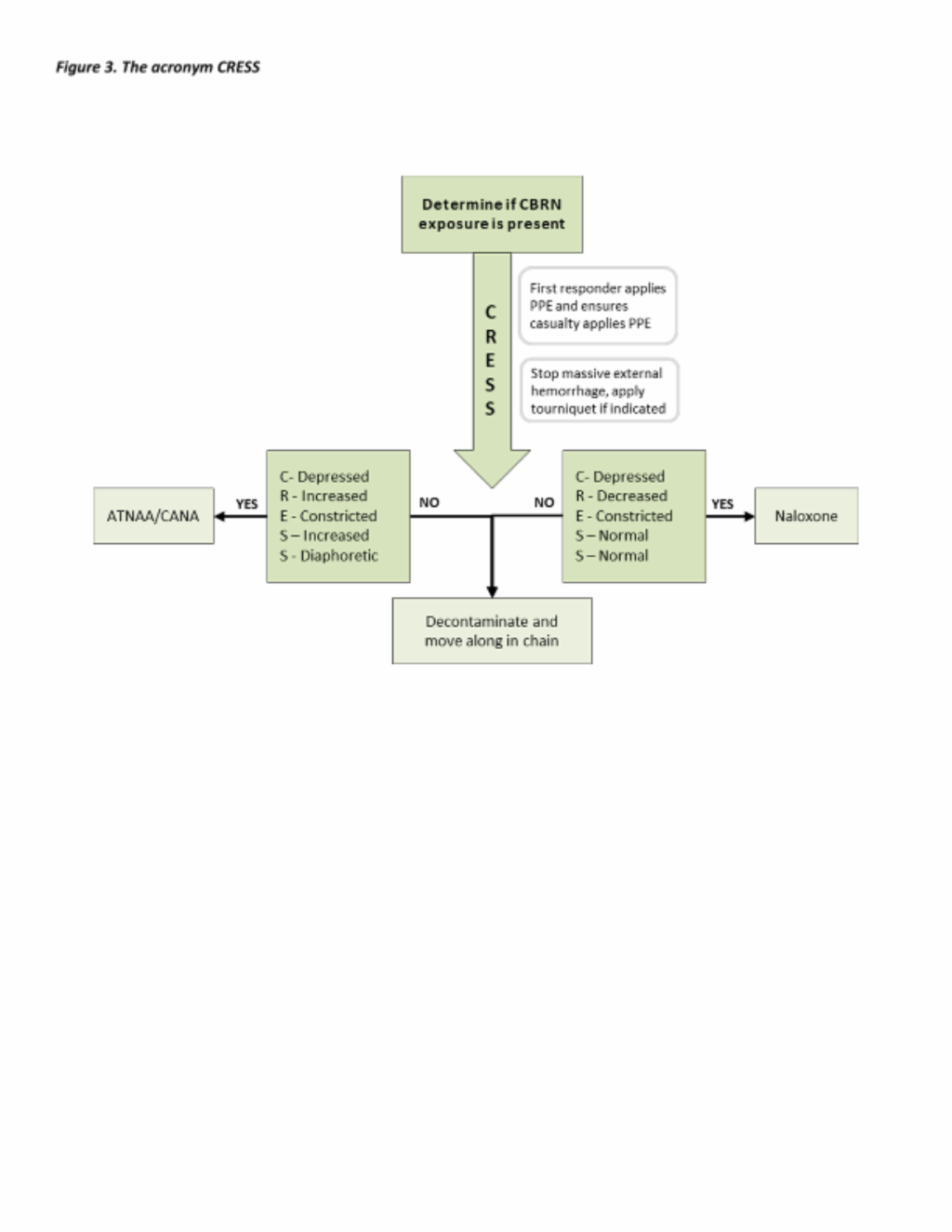
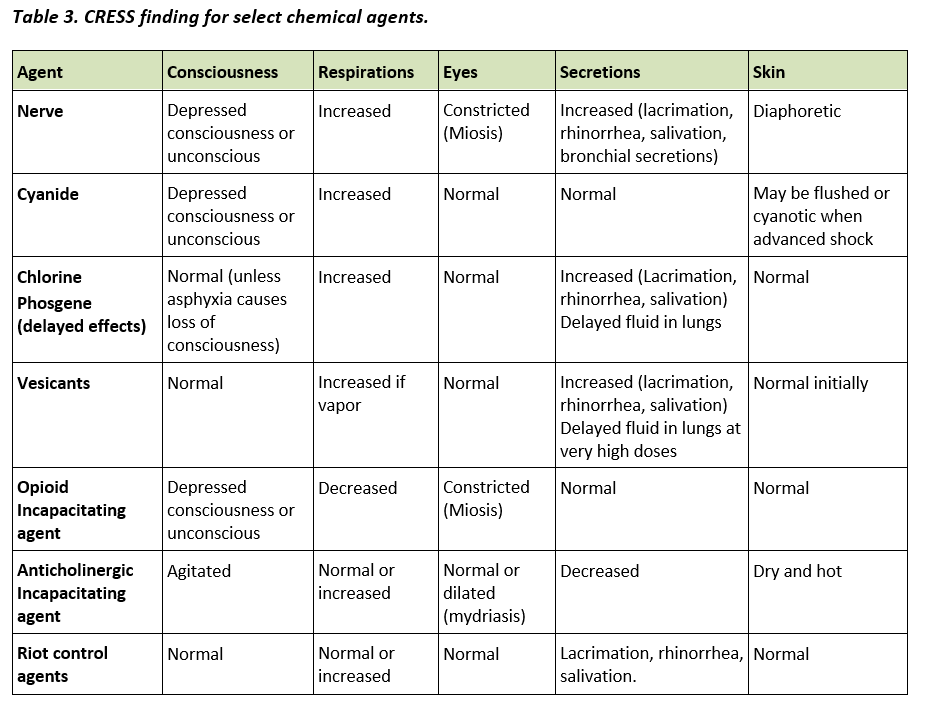
Just as trauma casualties present differently (blunt, trauma, GSW, blasts, etc.), CBRN casualties also have varied presentations. For example, chlorine casualties will require more attention to the toxic inhalation symptoms contrasted with a mustard casualty whose associated trauma may require more prompt intervention than the mustard-specific effects. CBRN casualties can be categorized by circumstances of exposure and presence or absence of trauma and CBRN effects. The acronym CRESS (Consciousness, Respirations, Eyes, Secretions, Skin) is employed to improve rapid identification of the type of chemical agent exposure.
In a CBRN-threat environment, casualties may have traumatic, CBRN or mixed injuries. Before applying TCCC, it is important to identify whether CBRN injuries are present and identify a suspected agent to allow first responders to protect themselves before treating the patient. While this often can be deduced using intelligence reports, technologic agent identification resources, and circumstances of the CBRN event, there will be times when agent identification will need to be made solely based on clinical assessment of symptoms. Some CBRN agents, such as nerve agents, can be rapidly fatal; therefore, recognition of such symptoms is the equivalent of identifying massive hemorrhage in the trauma patient. Clinical clues can be aggregated to identify the most likely agent responsible for symptoms according to the CRESS assessment. Each letter in CRESS corresponds to physical exam findings that can be used to categorize the suspected agent based on the constellation of findings. CRESS findings associated with specific chemical agents are shown in Table 3.
Figure 3. The acronym CRESS
The acronym CRESS is used to differentiate exposures to chemical agents. Exposure to biological agents is unlikely to cause early symptoms. Radiologic exposure may lead to early onset of symptoms (<1 hour post exposure) such as nausea, vomiting, diarrhea, and fever with high dose exposures (>6 Gray). CRESS findings for select chemical agents are summarized in Table 3.
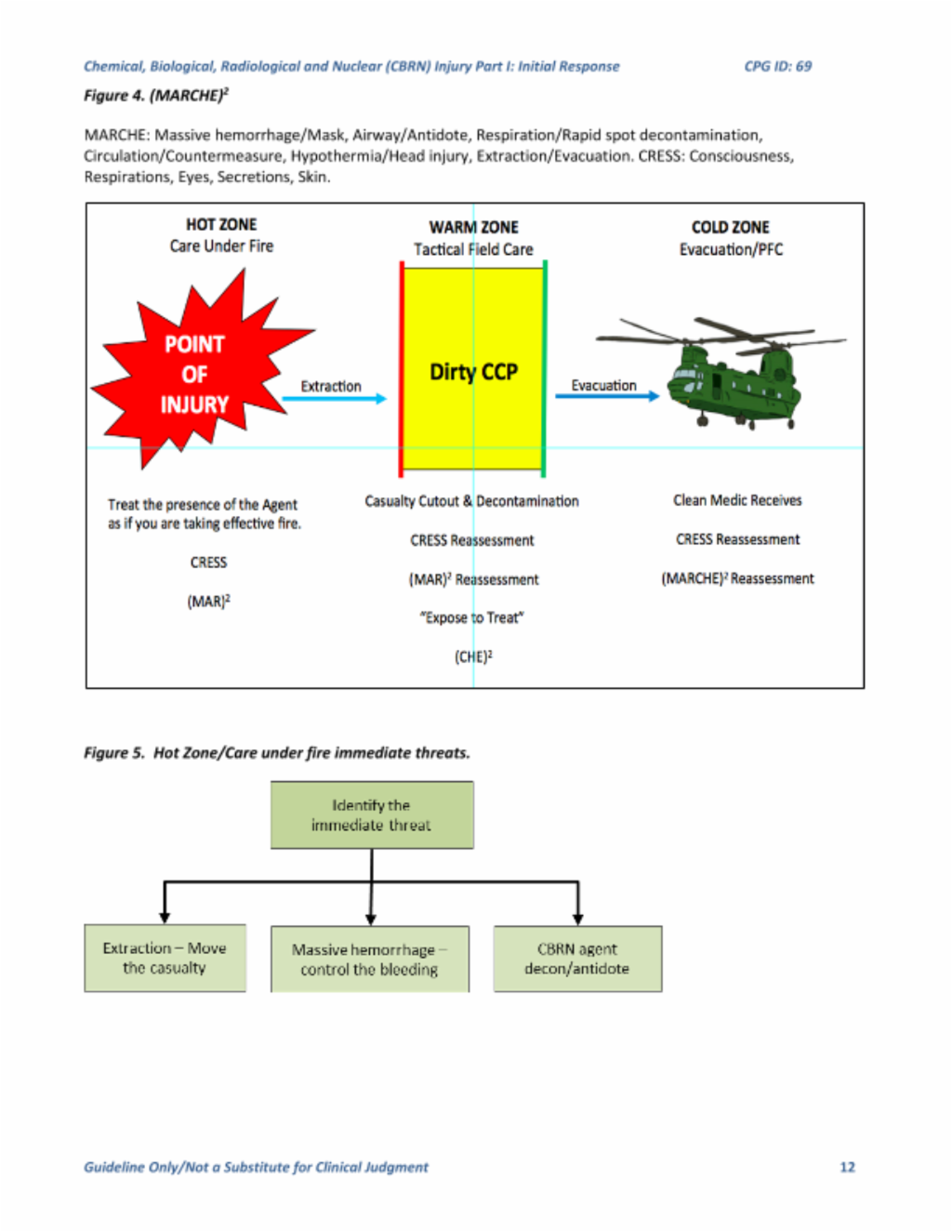
(MARCHE)2: Integrating TCCC MARCH with CBRN First Response
After initial assessment of casualty in CBRN-threat environment for the presence or absence of CBRN symptoms using the CRESS algorithm (and donning of PPE by first responders if present), the integrated assessment and management of TCCC and CBRN injuries can proceed.
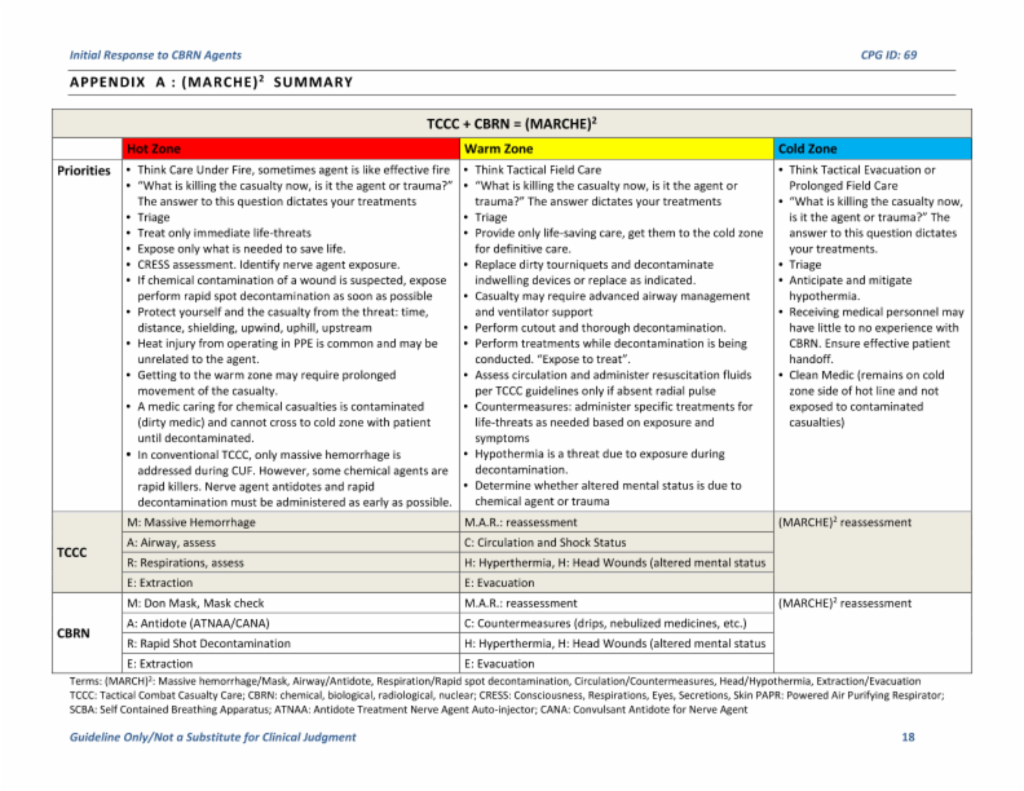
The acronym (MARCHE)2 integrates these two processes using the established TCCC MARCH algorithm (Massive Hemorrhage, Airway, Respirations, Circulation, Hypothermia) with CBRN priorities of Mask, Antidotes, Rapid spot decontamination, Countermeasures, Extraction and Evacuation. Combining these two approaches gives the acronym (MARCHE)2 or “MARCH-squared.”
Similar to TCCC, (MARCHE)2 can be broken into phases of care from highest threat (care under fire or active CBRN threat), to intermediate threat (tactical field care or warm zone casualty management), to lowest threat (PFC/evacuation or care of decontaminated casualties in cold zone). Figure 4 below.
Putting It All Together, Integrating CRESS and (MARCHE) 2
Step 1: Hot Zone/Care Under Fire
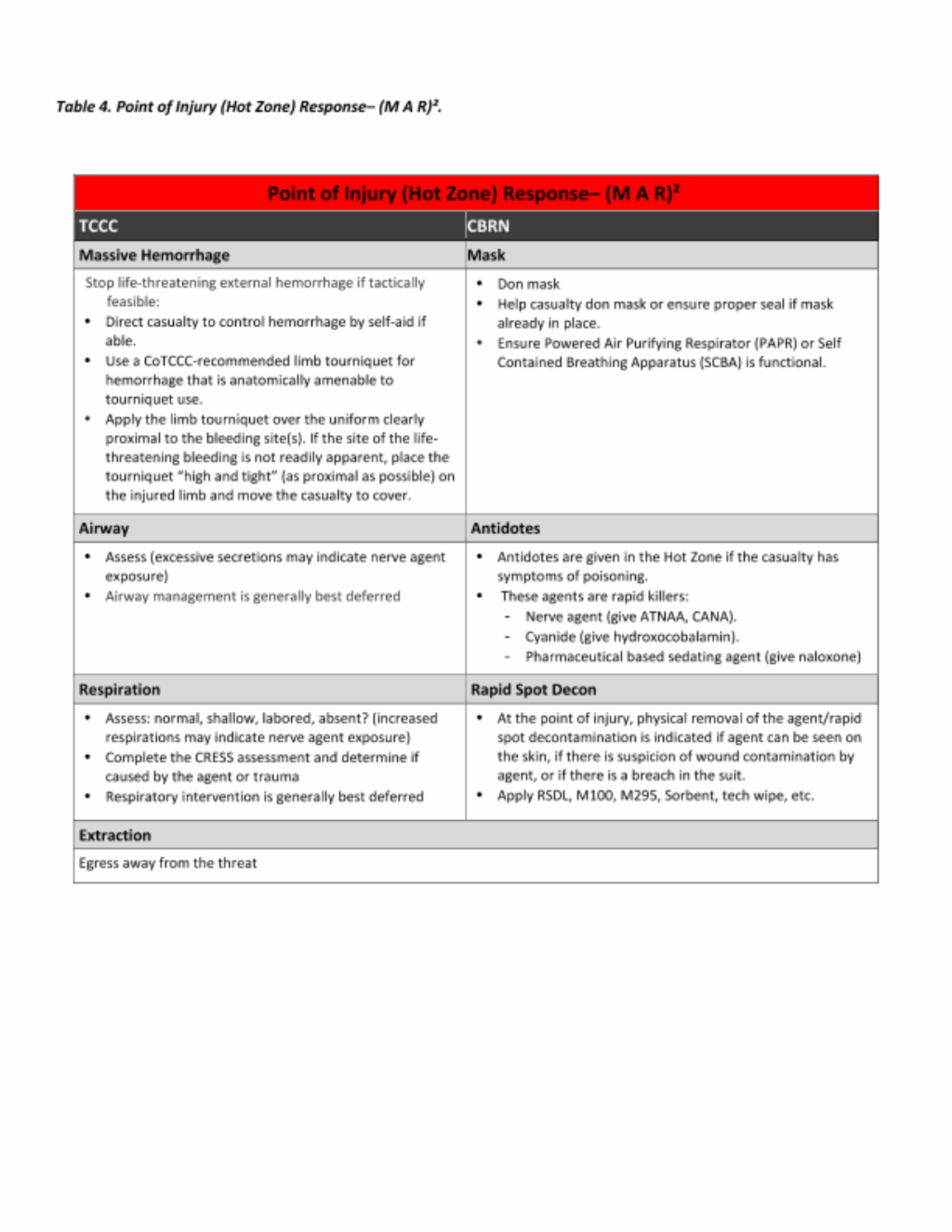
During “hot zone” care, the agent itself is similar to enemy fire. As such, the priorities are protection from and egress away from the threat (CBRN agent) for both casualty and provider. Both casualty and provider should don protective mask. If the casualty is incapacitated, the provider must ensure the casualty’s protective gear is applied and functional.
Only rapid interventions for immediate life-threats should be addressed. Massive hemorrhage, if present, may be the most immediate life threat and control of hemorrhage supersedes other interventions. Massive hemorrhage, if compressible, should be treated according to TCCC guidelines with limb tourniquets.
Perform a rapid assessment of the airway and respirations. Excessive secretions and increased respirations may indicate nerve agent exposure. Completion of the CRESS assessment will help determine if the symptoms are due to trauma or chemical agent exposure. Most airway and respiration interventions should be deferred. Occasionally, such interventions may take priority over mask application, but the decision to unmask a casualty in order to provide the intervention needs to be weighed against the risk of the contaminated environment.
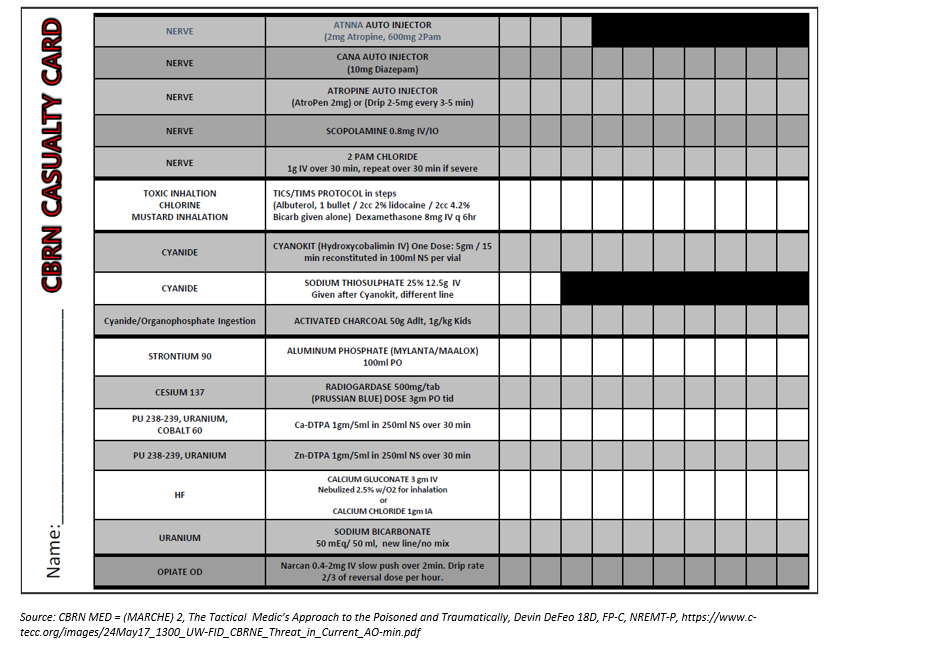
Some CBRN agents are rapid killers. The CRESS assessment will quickly determine whether immediate antidotes are required. If nerve agent exposure is suspected (decreased level of consciousness, increased respirations, constricted pupils, increased secretions, diaphoretic skin) then the provider should direct self-administration of Antidote Treatment Nerve Agent Auto-injector (ATNAA) and Convulsant Antidote for Nerve Agent (CANA) or administer the antidotes for an incapacitated casualty.
Rapid spot decontamination of skin or wounds is indicated when there is gross contamination on the skin or wounds or when protective gear is breached. If wound contamination with chemical agent is suspected, immediately expose the wound to perform rapid spot decontamination. This step is necessary even in a contaminated environment and may be lifesaving.
Treatment for cyanide can be considered in the hot zone, but the need to establish IV/IO access to administer hydroxocobalamin makes this a judgment call weighed against the time needed to reach the warm zone and the ongoing threat in the hot zone location. Naloxone 2 mg intramuscular or intranasal for opioid incapacitating agents may also be considered for life-threatening respiratory depression. As in TCCC, non-essential interventions should be deferred until the next phase of care.
Step 2: Warm Zone/Tactical Field Care
In the warm zone, or tactical field care phase, attention is given to decontamination and reassessment of the casualty. This phase occurs at a dirty CCP (hotline) and requires personnel dedicated to triage, decontamination, and patient treatment. At this phase, interventions may have altered the clinical presentation of the casualty (e.g., the patient may have decreased secretions and reduced airway resistance from use of the ATNAA), so it is important to take into account prior interventions and changes in the clinical status of the casualty. However, the goal is to quickly transition the casualty to the cold zone for definitive care, so only life-saving treatments should be done in the warm zone.
Decontamination and treatment can be synchronous processes. Medical personnel will need to clearly communicate with non-medical personnel responsible for decontamination. “Expose to treat” is used by decontamination personnel when the provider deems it in the best interest of the casualty to remove PPE to provide life-saving medical intervention. For example, the mask may be removed and the head, face, and chest quickly decontaminated so that the provider can ventilate the casualty and insert a sternal IO if parenteral antidotes, blood product or fluid resuscitation or countermeasures are immediately indicated. It would also be appropriate to prioritize decontamination of an arm to establish an IV or proximal humerus IO access.
Circulation should be assessed to identify external hemorrhage and shock. The provider can assess the effects of both CBRN agents and antidotes on the patient’s circulatory status. As decontamination ensues, dirty treatments are replaced with clean. Due to cold water and the need to fully expose the casualty in order to conduct thorough decontamination, hypothermia is a significant risk. Ensure rapid transit through the decontamination line with careful attention to hypothermia mitigation in order to prevent iatrogenic injury from the decontamination process. Additionally, personnel working within the warm zone in full protective gear are at risk of heat injury and stressors associated with operating in PPE, which must be recognized and mitigated
Step 3: Cold Zone/Tactical Evacuation Care/Prolonged Field Care
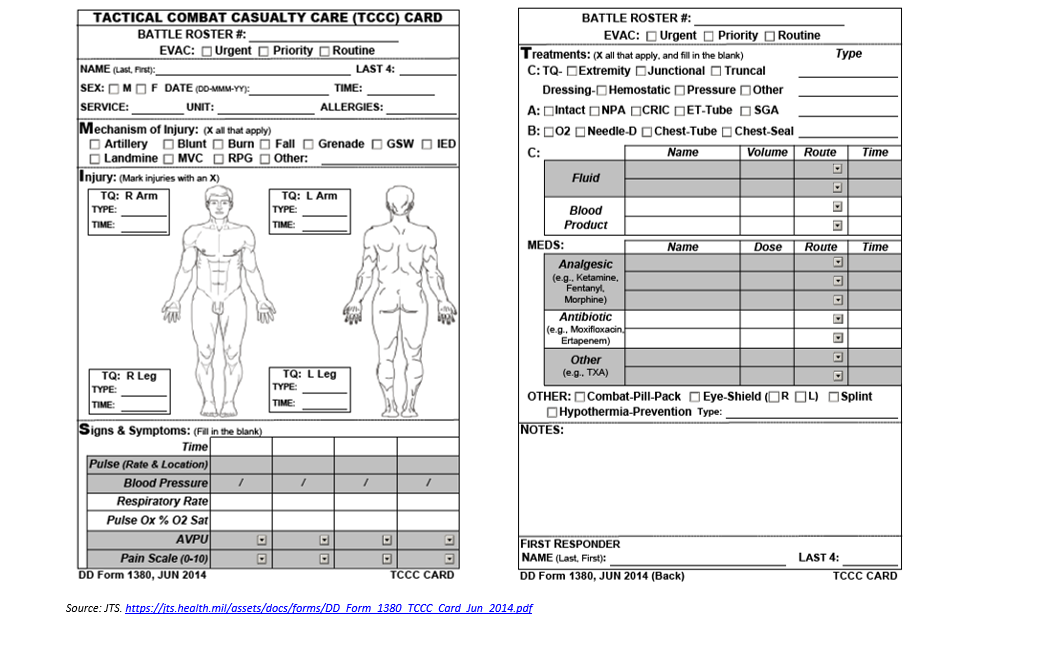
Once the decontaminated casualty has passed into the cold zone, they should be re-evaluated and receive needed interventions to include tactical evacuation or prolonged field care. The decontamination process alone can result in significant change in the casualty’s condition, so re-triage and reassessment are the first priority. If a secondary survey has not been performed, it should be done immediately after re-assessment of the primary survey. Documentation throughout the phases of care is vital to facilitate information transfer as the patient transits from one phase to another. The CBRN casualty card (Appendix B) is a useful tool to ensure comprehensive documentation relevant to the CBRN casualty. In the absence of a CBRN casualty card, the TCCC casualty card is sufficient if duration of care before transfer is short and patient is relatively stable. If handoff to higher echelon of care is delayed or patient is unstable, PFC documentation will likely be necessary. (Loos 2018)
It is important to take into consideration that receiving providers at all roles of care may have minimal to no experience with CBRN patients. Communication is crucial to ensure receiving providers understand previous decontamination and care and exposures that may be suspected. This can prevent delays in patient care caused by unnecessary repetitive decontamination or redundant treatments.
Casualties that reach the cold zone have been decontaminated and are now suitable for the full spectrum of care appropriate to the clinical environment and capabilities. There are however, some unique concerns to address in a combination CBRN/trauma casualty. While thorough decontamination and complete removal of a casualty’s clothing eliminates almost all debris, it is possible the casualty may have retained foreign material or contaminated dressings in wounds. Such material may pose risk to treating personnel from off-gassing or secondary contamination. Additionally, this residual contamination may represent a continuing source of exposure to the patient via a route that bypasses the normal barriers of the skin resulting in rapid systemic distribution of the agent. Larger fragments should be removed from the wound using a “no touch” technique with surgical instruments, followed by placing the material and instrument in a sealed container with hypochlorite solution, minimizing the risk to providers. When possible, the contaminated wound should be further irrigated with clean water. Providers dealing with contaminated foreign material or dressings can wear 3 pairs of nitrile gloves. The outer glove should be discarded every 20 minutes. In the absence of suspected retained debris, the fully decontaminated patient in the cold zone can be treated as all other patients at that level of care. The extent of wound care provided will depend on prehospital resources available and transport time to surgical care.
Evacuation Considerations
The en route care approach to the combined CBRN/trauma casualty should not differ from POI through TACEVAC. The (MARCHE)2 mnemonic provides a helpful methodology for assessing and applying critical interventions during transport. The medical provider should perform regular reassessments, recheck all (MARCHE)2 interventions, and intercede where necessary; prioritizing those interventions that are life-saving. In general, physical examination capabilities are degraded during transport. These challenges are amplified in the CBRN environment when the medical provider and crew are wearing PPE. Training while in MOPP IV and practicing patient care maneuvers that may be hindered by this protective posture is paramount to successful care of the CBRN casualty.
References
- Agency for Toxic Substances and Disease Registry. Warfare and Terrorism Agents. https://www.atsdr.cdc.gov/substances/toxchemicallisting.asp?sysid=34. Accessed January 8, 2018.
- Army Medical Research Institute of Chemical Defense. Chemical Casualty Care Division. Medical Management of Chemical Casualties Handbook. 3rd ed. Aberdeen Proving Ground, Md: The Division; 2000.
- Centers for Disease Control. Emergency Preparedness and Response. https://emergency.cdc.gov/agent/agentlistchem.asp. Accessed January 8, 2018.
- DeFeo DR, Givens ML. Integrating chemical biological radiological and nuclear (CBRN) protocols into TCCC. JSOM. 2018;1:118-123.
- Dembek ZF, Alves DA, U.S. Army Medical Research Institute of Infectious Diseases. USAMRIID's Medical Management of Biological Casualties Handbook. 7th ed. Fort Detrick, Md: U.S. Army Medical Research Institute of Infectious Diseases; 2011.
- Goans RE, ARMED FORCES RADIOBIOLOGY RESEARCH INST BETHESDA MD. Medical Management of Radiological Casualties. Online Third Edition. 2010.
- Keyes DC. Medical Response to Terrorism. Philadelphia: Lippincott Williams & Wilkins; 2005
- Kumar V, Goel R, Chawla R, Silambarasan M, Sharma RK. Chemical, biological, radiological, and nuclear decontamination: Recent trends and future perspective. Journal of Pharmacy and Bioallied Sciences. 2010;2(3):220-238. doi:10.4103/0975-7406.68505.
- Loos P, Glassman E, Doerr E, et al. Documentation in prolonged field care. JSOM. 2018;1:126-132.
- Ramesh AC, Kumar S. Triage, monitoring, and treatment of mass casualty events involving chemical, biological, radiological, or nuclear agents. Journal of Pharmacy and Bioallied Sciences. 2010;2(3):239-247. doi:10.4103/0975-7406.68506.
- Tactical combat casualty care guidelines 170828: Dated 28 August 2017. Accessed at: https://deployedmedicine.com/market/11/content/40 on 5 Apr 2018.
APPENDIX C: Casualty Care Cards
APPENDIX D: Additional Information Regarding Off-Label Uses in CPGs
Purpose
The purpose of this Appendix is to ensure an understanding of DoD policy and practice regarding inclusion in CPGs of “off-label” uses of U.S. Food and Drug Administration (FDA)–approved products. This applies to off-label uses with patients who are armed forces members.
Background
Unapproved (i.e., “off-label”) uses of FDA-approved products are extremely common in American medicine and are usually not subject to any special regulations. However, under Federal law, in some circumstances, unapproved uses of approved drugs are subject to FDA regulations governing “investigational new drugs.” These circumstances include such uses as part of clinical trials, and in the military context, command required, unapproved uses. Some command requested unapproved uses may also be subject to special regulations.
Additional Information Regarding Off-Label Uses in CPGs
The inclusion in CPGs of off-label uses is not a clinical trial, nor is it a command request or requirement. Further, it does not imply that the Military Health System requires that use by DoD health care practitioners or considers it to be the “standard of care.” Rather, the inclusion in CPGs of off-label uses is to inform the clinical judgment of the responsible health care practitioner by providing information regarding potential risks and benefits of treatment alternatives. The decision is for the clinical judgment of the responsible health care practitioner within the practitioner-patient relationship.
Additional Procedures
Balanced Discussion
Consistent with this purpose, CPG discussions of off-label uses specifically state that they are uses not approved by the FDA. Further, such discussions are balanced in the presentation of appropriate clinical study data, including any such data that suggest caution in the use of the product and specifically including any FDA-issued warnings.
Quality Assurance Monitoring
With respect to such off-label uses, DoD procedure is to maintain a regular system of quality assurance monitoring of outcomes and known potential adverse events. For this reason, the importance of accurate clinical records is underscored.
Information to Patients
Good clinical practice includes the provision of appropriate information to patients. Each CPG discussing an unusual off-label use will address the issue of information to patients. When practicable, consideration will be given to including in an appendix an appropriate information sheet for distribution to patients, whether before or after use of the product. Information to patients should address in plain language: a) that the use is not approved by the FDA; b) the reasons why a DoD health care practitioner would decide to use the product for this purpose; and c) the potential risks associated with such use.
Download CPG in PDF

READ FULL PDF