Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for Hemorrhagic Shock
Joint Trauma System
Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for Hemorrhagic Shock
SUMMARY OF CHANGES
- Addition of treatment algorithm to include prolonged use of REBOA with partial aortic occlusion.
- Clarification on prolonged partial occlusion times for both Zone 1 and Zone 3.
- Addition of techniques to monitor above and below aortic balloon pressures.
- Addition of balloon and sheath management during resuscitation and balloon deployment.
- Addition of balloon deflation guidelines during prolonged partial REBOA use.
Purpose
This CPG reviews the range of accepted management approaches for Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) as a hemorrhage control adjunct in traumatic shock and post-traumatic cardiac arrest in combat casualties. Prior CPGs focused on the technique of complete aortic occlusion with shortened occlusion times secondary to the risk of increasing distal ischemia with prolonged use. In review of civilian data, the median occlusion time was found to be 40 minutes, significantly beyond the recommended 30-minute complete aortic occlusion distal ischemia limit with first generation complete aortic occlusion devices.1 Updated fourth generation REBOA devices allow for controlled partial flow past the site of aortic occlusion to allow for prolonged REBOA use beyond the previous ischemia time limitations. This guideline is meant to reflect the growing use of partial REBOA in the care of the injured patient. Recommendations for use in the military setting must consider the unique challenges of the deployed environment. Mission parameters, tactical situation, casualty’s physical location and evacuation capability also determine the capabilities available for combat casualty care. Mechanisms and patterns of injury as well as the availability and experience level of surgical resources and resuscitation teams all influence the care rendered. The optimal management is best determined by the clinician at the bedside. This document addresses the use of REBOA for traumatic hemorrhage.
Background
Background
- Hemorrhage continues to be a leading cause of preventable death on the battlefield. It can be broadly categorized as compressible or non-compressible, depending on its location. Non-compressible Torso Hemorrhage (NCTH) arises from trauma to the torso vessels, pulmonary parenchyma, solid abdominal organs, or the bony pelvis.2 Because NCTH is not amenable to control by direct pressure or extremity tourniquet application, it is particularly lethal.3
- Resuscitative Aortic Occlusion (RAO) affords distal hemorrhage control while increasing cardiac afterload and thereby maintaining coronary and cerebral perfusion pressure until (or while) hemorrhage control is achieved.4 RAO has traditionally required a left thoracotomy or laparotomy for aortic exposure.5-8 Resuscitative thoracotomy has a high mortality rate, due largely to the nature of the injuries and because this technique is not typically employed until after arrest.9-11 Nonetheless, data from combat theaters indicate that there is a reasonable probability of long-term survival and recovery following RAO in appropriately selected casualties as described in the JTS Emergent Resuscitative Thoracotomy (ERT) CPG.12-14
- REBOA is an alternative form of RAO for patients at risk of imminent cardiovascular collapse. It is performed through a common femoral artery approach without the need for thoracotomy. REBOA is best applied prior to cardiovascular collapse when the site of hemorrhage is below the diaphragm and no open thoracic intervention is otherwise indicated.15
- ERT allows management of thoracic injuries and manual cardiac compression and thus remains the procedure of choice for patients with significant thoracic or cardiac injury. However, REBOA has been used in combination with open thoracotomy and/or sternotomy as a resuscitative bridge to open surgical control of hemorrhage to treat thoracic great vessel injury.16
- ERT may improve cardiac index as well as coronary and cerebral perfusion pressure compared to closed chest compression.17 However, when closed chest compressions are combined with REBOA, cardiopulmonary resuscitation is more effective allowing for higher end-tidal carbon dioxide (EtCO2) and cardiac compression fraction compared to open cardiac massage and aortic cross clamping.18
- RAO poses a significant risk of life-threatening and limb-threatening complications. RAO is a time-critical intervention that should never be undertaken without expedient access to definitive surgical hemorrhage control and without adequate vascular access for simultaneous resuscitation with blood. 2–20,8-17,19-30,19,22,25
- The major rate limiting step with REBOA is accurate and expedient common femoral artery (CFA) access. Ultrasound guided access is the preferred method for CFA access; however, in early experience, up to 50% of cases required open exposure. Smaller access sheaths are associated with improved outcomes.31-34 As early CFA access has become more routine in select trauma centers, the need for open CFA exposure has decreased to below 10%.35
- Literature for REBOA are mixed; some demonstrate a survival benefit20-21 while others suggest REBOA may actually worsen mortality.22-23 The advent of the wireless ER-REBOA™ and COBRA-OS®, combined with a better understanding of REBOA indications led to recent studies demonstrating the non-inferiority of REBOA. In patients that do not require cardiopulmonary resuscitation (CPR), REBOA has now shown a survival benefit.24-27,15 In 2018, a prospective analysis documented that REBOA improved survival beyond the emergency department and to hospital discharge compared to ERT when applied prior to traumatic cardiac arrest in patients with hemorrhagic shock.15
- A primary limitation of first generation REBOA (ER-REBOA-PLUS™ and COBRA-OS®) is the short Zone 1 aortic occlusion time prior to the increasing risk of distal ischemia (See page 10 for an Illustration of the zones of the aorta). The AAST AORTA database revealed that 63% of REBOA cases exceeded 30 minutes in Zone 1 and the median occlusion time was 40 minutes.1 Partial REBOA (pREBOA) addresses some of these limitations by allowing a small volume of blood to continuously flow past the balloon and perfuse distal tissues and organs while mitigating ongoing hemorrhage. The patient selection and indication guidelines for pREBOA are largely based on consensus guidelines from select civilian trauma centers.36,75,77
- In a preclinical study done by Polcz et al. 2021, Targeted Regional Optimization (TRO), a partial REBOA strategy, enhanced blood flow to vital organs like the heart and brain while maintaining a minimum level of perfusion to the lower body and limbs. Furthermore, partial occlusion facilitated faster renal function recovery. Finally, a linear correlation between distal Mean Arterial Pressure (MAP) and distal aortic flow rate was observed, providing a valuable clinical metric for informed decision-making.37
- Third generation pREBOA-PRO™ devices allow prolonged partial aortic occlusion in Zone 1 for at least 2 hours without increase in ischemic complications.38 In animal and civilian studies, it has been shown that Zone 1 partial occlusion can increase to 4 hours with treatable distal ischemic changes.39 In civilian trauma centers utilizing this technique, partial REBOA use has reduced cardiac arrest and blood product usage, and increased use of angioembolization with reduced distal ischemia.40
- Critical hemorrhage models in swine demonstrate that pREBOA can extend aortic occlusion time to at least 2 hours with continuous controlled pulsatile distal pressure of at least 20 mmHg systolic blood pressure (SBP) without increased distal ischemic complications.39 Clinical data in civilian trauma centers and early military data from the Ukraine War41-42 demonstrate similar results. Partial REBOA with an extension of occlusion time of up to 4 hours without compromising survival or worsening ischemic injury may be feasible.
- With increasing REBOA availability and provider experience, REBOA has successfully been utilized in multiple austere military locations.43-47 In austere resuscitations, REBOA use has facilitated blood product conservation, and been used as an adjunct during damage control surgery, creating a “bloodless” environment.45,47-48,79
- To our knowledge pREBOA has not yet been utilized in the deployed environment. To successfully perform pREBOA in the civilian hospital setting, significant team training is required to perform it effectively. Similarly, performing REBOA in deployed or austere settings requires significant team training.
Current Recommendations
- For the purpose of this CPG, the use of REBOA forward deployed requires training of all team personnel and expedient access to a damage control surgery capability.
- Partial REBOA is conditionally preferred over complete REBOA. This requires training of all surgical and damage control resuscitation team personnel and acquisition of the partial occlusion device. The pREBOA-PRO™ catheter is currently the only FDA-approved catheter for partial aortic occlusion.
- For accurate titration of partial REBOA, central arterial blood pressure should be measured above and below the balloon, this requires a two arterial line transducer set up. A target central aortic SBP of 90-110 mmHg above the balloon, and minimum pulsatile SBP of 20 mmHg below the balloon, while titrating distal pressure to a target systolic blood pressure of 20 - 50 mmHg is desirable in partial occlusion.49 Of note: the pREBOA-PRO™ catheter provides the ability to measure above and below the catheter without additional access.
- Partial aortic occlusion of Zone 1 should ideally be limited to 2 hours, and Zone 3 should be limited to 4 hours. This continues to be an active area of clinical and animal research at the time of publication of this CPG.
- Complete aortic occlusion should be limited to less than 60 minutes in Zone 1, optimally < 30 minutes. When placed in Zone 3, keep RAO < 60 minutes.
- The implementation of REBOA must be determined at each site based on training, experience, local resources, expedient availability of a damage control surgery capability, and evacuation timelines.
- Documentation of Aortic Occlusion via open thoracotomy or REBOA will be done using the Aortic Occlusion (AO) Procedure Note that is found in Appendix F of this CPG.
Use of REBOA In Traumatic Arrest & Profound Shock
Indications for the use of REBOA are summarized below. These indications mirror the indications for resuscitative thoracotomy with the exception that shock or arrest secondary to penetrating chest trauma is a contraindication to REBOA (See the JTS Emergency Resuscitative Thoracotomy, 18 Jul 2018 CPG).14
Due to the mixed literature supporting the use of REBOA, it is imperative that REBOA only be considered in the appropriate patients with access to rapid definitive hemorrhage control, placed by trained providers, and with medical/surgical support personnel facile not only in setting up and managing REBOA and its required equipment, but also the care of the patient (both while the REBOA is in place and after removal of the balloon and its arterial sheath). REBOA is only a bridge to definitive hemorrhage control; therefore, all these variables necessitate consideration.
It is also important to be mindful that REBOA is a temporizing measure, and once the balloon for aortic occlusion is inflated, surgical capabilities must be available with definitive hemorrhagic control achieved within a maximum of 60 minutes since inflation (Partial: Zone 1 –2 hours, Zone 3 – 4 hours).
Patients where REBOA can be considered:
- Traumatic arrest without evidence of thoracic, neck, or upper extremity hemorrhage (closed cardiac compressions must concomitantly be performed).
- Severe hemorrhagic shock or suspected impending traumatic arrest with non-compressible truncal hemorrhage below the diaphragm.
- Complex lower extremity amputations (high thigh injuries) where tourniquets and junctional tourniquets have been ineffective at hemorrhage control.
Initial management priorities for patients with traumatic arrest or impending arrest include early control of hemorrhage and hemostatic resuscitation as described in the JTS Damage Control Resuscitation CPG.18,80 The initial focus in patients presenting in profound hemorrhagic shock, to include loss of pulses, is to determine the best resuscitative strategy, and whether resuscitation is appropriate or futile in a moribund patient. The following must be rapidly determined:
- Mechanism and pattern of injury
- Presence of a pulse
- Duration of cardiac arrest
- Presence or absence of an organized, narrow complex cardiac rhythm and/or organized cardiac activity by ultrasound
- Resources available
- Availability of surgical capability for definitive hemorrhage control
- Number of concurrent casualties
Patients exsanguinating from abdominal, pelvic, or junctional lower extremity bleeding may be candidates for REBOA. Such patients are identified by penetrating mechanism of injury to abdomen or pelvis, blast or blunt mechanism with positive FAST or suspected pelvic fracture, or massive proximal lower extremity trauma with signs of impending cardiovascular collapse.
Exsanguinating hemorrhage in the chest must be ruled out prior to placing REBOA—this can be done with bilateral chest tube placement, x-ray, or thoracic ultrasound. In cases of major chest hemorrhage, occlusion of the aorta may increase thoracic bleeding and is thus best addressed via thoracotomy or sternotomy.
A decision algorithm for (RAO) is found in Appendix A. If RAO is performed, concurrent hemostatic resuscitation and closed chest cardiac massage must continue while the procedure is performed.50 If RAO is not performed, resuscitative efforts should cease unless there is a compelling reason to consider a non-traumatic arrest.
The gold standard for aortic occlusion in traumatic arrest remains a left anterolateral thoracotomy (See JTS Emergent Resuscitative Thoracotomy CPG).
Trans-abdominal Aortic Occlusion
The aorta can also be occluded trans-abdominally at any point along its length. It can be occluded with either application of a clamp, or compression with a retractor or the surgeon’s hand. Alternatively, if there is limited surgical assistance or a need to reduce the number of instruments in the upper abdomen, a balloon aortic occlusion at Zone 1 or Zone 3 can be considered, depending on where the focus of bleeding is located.
As with all other forms of RAO, restoration of aortic perfusion should be carefully coordinated with the rest of the team to minimize the effects of reperfusion and blood volume shifts.
It should be noted that reperfusion after partial balloon occlusion can occur with gradual titration of volume while monitoring the response of above and below balloon pressures over 10 - 30 minutes to minimize negative hemodynamic consequences during reperfusion. With complete occlusion REBOA catheters, it is not possible to gradually titrate stable reperfusion as the balloon configuration essentially provides “all or none” occlusion.
REBOA can be considered in 6 sequential steps:
- Arterial access and positioning of sheath
- Positioning of the balloon
- Inflation of the balloon
- Establish degree of aortic occlusion (partial vs complete)
- Operative/procedural control of bleeding
- Deflation of the balloon
- Sheath removal
REBOA can be performed preemptively in patients with high-risk injury patterns and unstable physiologic parameters as described above. In this way, REBOA can be proactive rather than reactive in appropriate patients. The indications for REBOA are summarized in Appendix A for traumatic arrest and Appendix B in cases of profound shock. If proximal aortic occlusion is required, this is termed Zone 1, whereas distal aortic occlusion is termed Zone 3. Zone 1 REBOA deployment will be used in most patients presenting with hemorrhagic shock, and may be used in all patients with traumatic arrest, regardless of injury pattern, due to the benefits on a patient’s MAP.51,81
In clinical situations where REBOA is being considered, early placement of an arterial line in the common femoral artery (CFA) is recommended. CFA access has consistently been identified as the rate limiting step to REBOA deployment.32 Obtaining early CFA access in the form of an arterial line can greatly decrease REBOA placement time: an existing common femoral arterial line can quickly be re-wired and upsized to a 4 - 7 Fr sheath (depending on what system is used) for REBOA in the event of patient deterioration. It can also be used to transduce the distal SBP with the pREBOA-PRO™ partially inflated.
Goal proximal SBP in REBOA is between 90 - 110 mmHg. If there is concern for traumatic brain injury a SBP > 110 mmHg has been advocated (See the JTS CPG Traumatic Brain Injury and Neurosurgery in the Deployed Environment CPG).53 If utilizing (MAP goals instead of SBP, it is recommended to maintain MAP of 55 - 65 mmHg proximal to the balloon. Only after that range is achieved does the user consider if the patient's physiology requires partial or complete occlusion.
Zone 1: ~46cm
Zone 3: ~28 cm
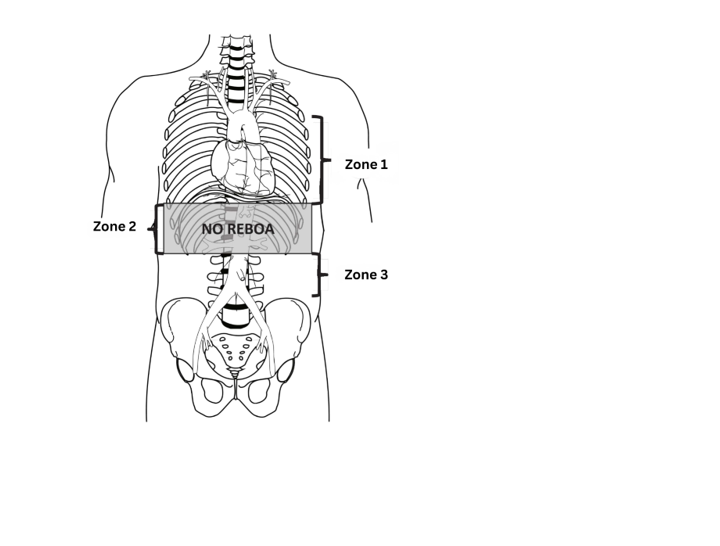
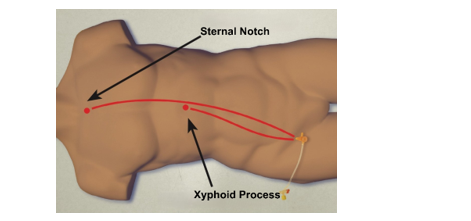
- Zone 1: Placement of aortic balloon in the descending thoracic aorta (insert catheter to Zone 1 markers (45 - 49 cm at the level of the sheath) or measure the balloon with P tip from sternal notch to arterial sheath).
- Consider using for suspected infradiaphragmatic hemorrhage with high suspicion for intra-abdominal hemorrhage (positive FAST, significant mechanism of injury that involves the abdomen).
- Zone 2: DO NOT PLACE REBOA IN THIS ZONE—placement here risks injuring or thrombosing mesenteric vessels supplying abdominal visceral organs.
- Zone 3: Placement of aortic balloon directly above the aortic bifurcation (insert catheter to Zone 3 markers [26 - 29 cm] or measure P-tip from the xyphoid process to arterial sheath). Only use when intra-abdominal hemorrhage has been ruled out (negative FAST or mechanism/injury pattern that is not consistent with a concomitant abdominal injury). Consider use for: pelvic hemorrhage, junctional hemorrhage, profuse hemorrhage from high traumatic lower extremity amputations not controlled by tourniquets.
Zone 1 REBOA – 60 minutes maximum, optimally < 30 minutes.
Zone 3 REBOA – 60 minutes.
Preclinical research has shown a Zone 1 complete aortic occlusion time of 60 minutes or more results in significant metabolic derangement and organ damage that may negate any benefits obtained by its assistance with hemorrhage control.54,74 In comparison, complete occlusion time of 30 minutes or less significantly improved outcomes without evidence of severe physiologic costs.
Zone 3 REBOA historically has been considered acceptable for up to 4 - 6 hours;52-55 however, recent analysis in preclinical models have led to the revised recommendation to target Zone 3 balloon occlusion times of no greater than 60 minutes.55-57,58
Zone 1 – 2 hours; Zone 3 – 4 hours
Distal SBP => 20mmHg (20 - 50 mmHg) or MAP ~20 mmHg
Partial aortic occlusion is defined as having partial blood flow past the balloon to minimize the risk of ischemia with longer occlusion times. In the absence of measuring flow, the best clinical surrogate is blood pressure. To accurately control inflation, the user will need to monitor the pressures proximal and distal to the inflated balloon. Two arterial line transducers and set ups are required to accomplish this. Similarly, during prolonged aortic occlusion, monitoring blood the proximal and distal pressures is necessary to determine the extent of partial occlusion. The distal mean arterial pressure is more accurately correlated with flow below the balloon than the above the balloon pressure.59 The minimum recommended distal SBP considered to be a successful partial occlusion is 20 mmHg,39 with a desirable target systolic blood pressure range of 20 - 50 mmHg.49 If utilizing MAP instead of SBP it is recommended to maintain a MAP of 20 mmHg (below the balloon) since MAP and SBP are very close in this low-pressure range. This increases the occlusion time in Zone 1 to at least 2 hours and Zone 3 to at least 4 hours in both preclinical and clinical data.38-39 Due to the semi compliant design of the pREBOA-PRO™ catheter, several investigators have documented that inflation of the balloon does not need to be adjusted after initial setting of below MAP.39
The provider, or assistant, should promptly document placement time, pre-/post-placement blood pressure and MAP, and REBOA insertion distance. Use the Aortic Occlusion (AO) procedure note that is found in Appendix F for specific REBOA documentation. Whether using a complete or partial occlusion catheter, balloon volume and inflation time should be noted at the insertion site for reference by all providers caring for the patient. The provider is responsible for prevention of catheter migration, particularly during patient transport. A provider who is knowledgeable about the management of REBOA should attend to the patient while awaiting definitive surgical repair, to include transport. The trained provider is responsible for ensuring a safe and competent hand off.
Due to its placement in a pulsatile vessel, the REBOA will migrate without properly securing it. In addition to suturing the femoral arterial sheath to the skin, the REBOA catheter must be secured at the appropriate distance marker either by hand or with a suture to the skin tied directly to the catheter to keep the catheter in place at the appropriate distance marker, especially during transport.
The arterial line for the REBOA is narrow and can thrombose easily. Frequent flushing of the arterial line by medical personnel is often necessary. Careful and diligent monitoring of the arterial waveform is necessary: if it appears the arterial waveform has dampened, flushing of the arterial line may be necessary. Often, it may be helpful once the patient is better resuscitated to place a radial arterial line to continue to monitor proximal blood pressure in the event that the REBOA arterial line thromboses, malfunctions, and/or can no longer transduce.
The balloon should be deflated once definitive hemorrhage control has been obtained. Communication with the assistant holding the apparatus securing the catheter and the anesthesia team is critical before consideration of deflating the balloon. When deflating the balloon, turn the three-way stopcock and withdraw saline slowly and deflate the balloon slowly. A good rule of thumb is to deflate the balloon 1 ml every minute. During and after balloon deflation, the team should be prepared for hemodynamic changes related to reperfusion, washout of metabolic byproducts, and acidosis. Ensure adequate blood product resuscitation prior to balloon deflation.
Complete Occlusion:
If using complete occlusion catheters (ER-REBOA-PLUS™, COBRA-OS®, etc.) this step can be anticipated to result in a significant decrease in afterload and hypotension and may result in cardiac collapse. Additional resuscitation may be needed even with slow balloon deflation. The user can anticipate approximately a 10% change in flow past the balloon during deflation with as little as 0.2 ml of fluid removal. Intermittent balloon inflation and deflation may be necessary during ongoing resuscitation until hemodynamic stability is restored.
Partial Occlusion:
If using pREBOA-PRO™ the user is advised to gradually remove fluid from the balloon every 10 minutes to increase the distal SBP by 20 mmHg. These small adjustments in flow over time should mitigate the ischemic reperfusion changes often encountered with removing an aortic clamp or deflating a complete occlusion aortic balloon. This slow deliberate deflation method will minimize the need for reinflation unless further hemorrhage is encountered.
During catheter removal, ensure that the balloon is fully deflated. If significant resistance is felt as the catheter is being removed, it is likely that the balloon cannot pass through the sheath. In this case, the balloon and the arterial sheath must be removed together. See below on sheath management and removal.
Sheath Management and Removal:
After placing a REBOA, careful management of the femoral sheath is imperative. The majority of complications associated with REBOA use are related to the sheath and access site complications. Reported femoral access complications include arterial disruption, dissection, pseudoaneurysms, hematoma, thromboembolic phenomenon, and extremity ischemia. These complications have resulted in limb loss.29,30
Due to the risk of sheath dislodgement or vessel wall damage, excessive patient movement should be avoided. Patients with indwelling sheaths should be positioned supine or reverse Trendelenburg only. If the patient must be moved or turned, they should be kept in a flat position and log rolled. Avoid flexing the hip.
This may be performed either at the Role 3 facility or as soon as possible after arrival at a Role 4 facility, depending on resources available to perform and interpret the ultrasound.
Once definitive hemorrhage control has been obtained, the REBOA sheath should be removed.
If arterial pressure monitoring is still required, perform at an alternate arterial line site. Prior to removal, an angiogram through the sheath to document distal limb perfusion is best practice, though not always available. If a large sheath size is used, a patient is coagulopathic, or there is technical difficulty in sheath removal, a cut down and arterial repair, patch or graft may be required. This may be best accomplished in the Role 3 environment with access to specialists and/or surgical backup.26
When there is concern for re-bleeding, the sheath may be left in place without aortic occlusion. By leaving the sheath in place, the REBOA can easily be reinserted, and aortic occlusion can quickly be obtained if rebleeding occurs or hemorrhage continues.60,76 In general, and situation/resource dependent, the sheath should be left in place during any active or ongoing resuscitation. The sheath should not be removed immediately prior to transport and is best removed where vascular complications can be treated and managed.
Even in an austere environment, protocols for use and follow on care should be planned and discussed prior to implementation. Team training and awareness of pitfalls are critical to ensure the best possible outcomes.
Aeromedical Evacuation Considerations
Patients who receive REBOA at a Role 2 and need to be evacuated to a higher level of care should have hemorrhage control addressed, and balloon deflated prior to transfer.
Under no circumstance should a Zone 1 complete occlusion REBOA remain inflated during transport. In rare situations when a short-distance rotary-wing evacuation to higher level of care is possible, a Zone 3 REBOA inserted at Role 2 may remain inflated during transport, however, this requires exceptional communication and planning to avoid undue risk of ischemic injury.
Partial aortic occlusion has prolonged treatment times to at least 2 hours in Zone 1 and at least 4 hours in Zone 3. It is now considered feasible to transport a patient with a provider trained in partial REBOA if it is expected to arrive at the location to provide definitive hemorrhage control within these time recommendations.
If transport is available, a medical provider trained in hemodynamic monitoring and manipulation of the occlusion balloon should always accompany the casualty. If a REBOA sheath is in place in a trauma patient, re-placement/re-inflation of the balloon during transport is an option for trained providers in the event of sudden profound hypotension. Simultaneous blood transfusion should be anticipated, and partial occlusion should be achieved as described above.
The essential equipment for REBOA is provided in Appendix G while the appropriate technical steps and considerations are summarized in Appendix C.
Training
Prior to using REBOA, providers should have a thorough knowledge of the device, its indications, use and potential complications. For teams who do not routinely perform REBOA in clinical practice when not deployed, an organized curriculum-based REBOA training course should be completed before deployment where REBOA could be used. Organized, curriculum-based REBOA training courses such as the American College of Surgeon’s Basic Endovascular Skills for Trauma (BEST) course or the ‘Resuscitation Adjuncts: Prehospital Transfusion & REBOA’ (RAPToR) Course are available. Training can also be requested by emailing CPGtrainingrequest@PrytimeMedical.com. Successful completion of a REBOA training, including a didactic and hands on skills component, is recommended prior utilization of the device. Skills training can be achieved through high-fidelity simulation, perfused cadaver or live tissue training. Critical skills include access to the CFA with ultrasound and cut down, sheath placement and positioning, and REBOA operation and removal. Anatomically correct models are critical for accurate training of CFA access skills, and thus perfused cadavers are recommended to meet this requirement.61-63
Ultimately, the decision to perform REBOA on patients at high risk for hemorrhagic death will depend on the specific injury pattern, individual provider experience, team training, and local resources.
Partial REBOA Use By Non-Surgical Resuscitation Teams
Advanced resuscitation teams may be utilized in austere environments as a bridge to surgical hemorrhage control. Data on the effectiveness of this approach are lacking. However, the development of the partial occlusion REBOA catheter opens a window for potential use prior to surgical team handoff.
Use of partial REBOA in the austere, prolonged casualty care environment brings along its own complex set of problems. However, these problems, if planned for correctly, can be addressed. The use of in-line mean arterial pressure monitoring devices to monitor aortic pressures are crucial, as this provides the ability to monitor balloon effectiveness as well as the effectiveness of the resuscitation process. Obtaining the necessary insertion supplies (see Appendix G) as well as planning and training for these scenarios will ensure proper preparation should this need arise. Unit-specific plans should be assessed to maximize readiness. Planning for the use of partial REBOA in the prolonged casualty care (PCC) population should ensure all members of the team are in agreement and should only be considered by fully trained and equipped resuscitation teams. The standards laid out in this CPG should be the building blocks of the unit specific PCC plan of care.
Partial REBOA in this setting may be considered if all the following conditions are met:
- The casualty would otherwise die prior to reaching a surgical team without REBOA (NCTH, refractory hemorrhagic shock).
- The team is trained in partial REBOA therapy.
- Blood product resuscitation, preferably whole blood, is available but failing to resuscitate the patient.
- Time to surgical team handoff is less than 2 hours for Zone 1, less than 4 hours for Zone 3.
REBOA Pitfalls
- Making the decision to perform REBOA too late. Mortality is high after loss of pulses has occurred, as it is with ERT.
- Difficulty locating the common femoral artery in the groin. The clinician must be very familiar with open, percutaneous, and ultrasound guided femoral access techniques. Early CFA access is recommended even if REBOA not utilized.
- Insertion of the REBOA below the femoral artery bifurcation (into the superficial femoral artery). The catheter should be placed in the common femoral artery, just below the inguinal ligament (typically above the groin crease). Insertion into the superficial femoral artery is associated with an increased risk of thrombosis and limb loss.
- Failure to address chest pathology. Always evaluate the chest by X-ray, ultrasound, or bilateral chest tube placement to identify and treat significant hemothorax or pneumothorax. Convert to thoracotomy to address massive hemothorax.
- Failure to recognize complete aortic occlusion when partial occlusion is intended. This may occur when below-balloon pressures are not monitored.64 A validated miniaturized handheld pressure monitor is a convenient alternative to problematic traditional arterial line and monitoring apparatus especially in austere environments.64
- Unrecognized proximal femoral or iliac artery transection preventing endovascular access on the side of the injury. This may occur with penetrating pelvic trauma or severe pelvic fracture—check bilateral femoral pulses and access the side with a stronger pulse if there is a difference. Do not hesitate to switch to the opposite groin or convert to thoracotomy.
- Catheter or guidewire does not pass freely. This could indicate injury to the vessel. Do not inflate balloon. Consider accessing the opposite groin or convert to thoracotomy.
- Over-inflating the balloon. The ER REBOA-Plus™ balloon capacity is 24 ml. Zone 1 may require as little as 8 ml and Zone 3 as little as 2 ml to achieve occlusion. Over-inflation may rupture the balloon or injure the aorta. The pREBOA-PRO™ catheter comes equipped with an overinflation safety valve to minimize risk of increased balloon pressures (> 1 atm) that could cause vascular injury.
- Leaving the balloon inflated too long. Only 30 - 60 minutes of Zone 1 complete occlusion with the ER REBOA-Plus™ or COBRA-OS® balloon is advised, and the shorter the better. Achieve rapid control of bleeding sites with temporizing measures such as clamping to allow the earliest reperfusion; most suturing, ligating, solid organ removal, and vascular shunting may be done after balloon deflation. Death secondary to ischemic injury has been reported with longer complete occlusion times.
- Failure to work with a heightened level of urgency once REBOA is placed. Some patients may regain “stability,” however, complete balloon occlusion is just like a cross clamp, with the same complications of visceral and spinal ischemia. Every effort should be made to restore perfusion as soon as possible to limit ischemia.
- Failure to adequately secure the REBOA catheter after balloon inflation, resulting in migration of the balloon. The catheter position must be maintained during and after inflation to avoid distal migration until aortic pressure and pulsatility are restored.
- Deflating the balloon too quickly before adequate volume resuscitation. Ensure that the anesthesia team is prepared for reperfusion prior to balloon deflation.
- Premature removal of the arterial sheath. The sheath should remain in place if the patient is coagulopathic, may have ongoing bleeding in the abdomen or pelvis, or is being transported within theater to a higher level of care.
- Injury to the arterial access point. After removal of the sheath, monitor the instrumented leg closely for re-bleeding and thrombus/intimal injury. Decreased lower extremity perfusion may require further angiography, thrombectomy, or direct arterial repair.
- Committing multiple resources to a futile resuscitation. Anticipate massive transfusion, personnel required, surgical supplies, diversion of resources from more salvageable casualties, etc.
Future Considerations
A retrospective capability gap analysis of the UK Joint Theatre Trauma Registry suggested that as many as one in five severely injured casualties have wounds that may be amenable to treatment with REBOA.65 The development of the 4 Fr COBRA-OS® , 7 Fr ER REBOA-Plus™ catheter and subsequent versions facilitates insertion of the device and may lead to more widespread use of this approach in the austere environment. Specifically, the development of the novel semi compliant pREBOA-PRO™ catheter extends occlusion time and decreased management of the balloon volume.39 The feasibility of training non-physician caregivers to place REBOAs in the prehospital settings is being investigated.66-67 Research is currently being conducted to improve visualization tools regarding cannulation and targeted training of medical providers. Partial REBOA, intermittent REBOA, regional hypothermia, and pharmacologic adjuncts continue to undergo validation as a means of prolonging aortic occlusion time.68,69,70,78 Future animal studies with realistic models of injury are being developed to provide detailed multisystem organ assessment to accurately define organ injury and metabolic burden associated with prolonged partial REBOA application.71 Ongoing research seeks to identify modifications to the REBOA technique that may be required when it is combined with other resuscitation modalities such as tranexamic acid. Researchers are also striving to clarify patient selection, evaluating the impact of REBOA on thoracic injury, and traumatic brain injury.72 All of these advances should refine the optimal use of this resuscitation adjunct. Longitudinal data in the civilian and military setting will assist in defining the ideal clinical situation in which REBOA can be of maximal benefit.
Performance Improvement (PI) Monitoring
- Patients with injury to chest, abdomen, pelvis, and/or limb with SBP > 0 and < 90 or CPR in progress on arrival to first MTF AND not isolated head injury.
- Patients who received REBOA.
- REBOA is not performed in patients with no signs of life or CPR > 15 minutes or isolated severe TBI, penetrating neck injury, or penetrating extremity injury.
- If performed, REBOA was performed for the indication of hemorrhagic shock associated with abdominal, pelvic, or junctional lower extremity bleeding, or other indication is clearly documented.
- If REBOA performed, the patient was assessed for thoracic hemorrhage (EFAST or chest X-ray (CXR) results documented, or bilateral chest tubes placed).
- Blood pressure pre and post REBOA and balloon times (inflation and deflation) are documented in REBOA procedure note.
- Lower extremity pulses are documented hourly for 24 hours after balloon deflation.
- Patient did not have REBOA performed if patient had no signs of life or CPR > 15 minutes, isolated severe TBI, penetrating neck injury, or penetrating extremity injury.
- Patient had REBOA performed for hemorrhagic shock associated with abdominal, pelvic, or junctional lower extremity bleeding, or another indication that was clearly documented.
- Patient assessed for thoracic hemorrhage (EFAST or CXR results documented or bilateral chest tubes placed) prior to REBOA being performed.
- Patient had REBOA performed with complete REBOA procedure note to include documented blood pressure pre and post REBOA and documented balloon times (inflation and deflation).
- Patient had REBOA performed with lower extremity pulses documented hourly for 24 hours after balloon deflation.
- Patient Record
- Department of Defense Trauma Registry (DoDTR)
- Number of REBOA interventions, performance, and adherence measures will be reported quarterly by JTS PI Branch Chief to the JTS Chief.
- JTS will identify REBOA patients in the trauma registry and facilitate capture of complete medical records.
System Reporting and Frequency
The above constitutes the minimum criteria for PI monitoring of this CPG. System reporting will be performed annually; additional PI monitoring and system reporting may be performed as needed.
The system review and data analysis will be performed by the JTS Chief, and the JTS PI Branch.
It is the responsibility of the JTS PI Branch Chief to ensure system-level compliance with this CPG. It is the trauma team leader’s responsibility to ensure familiarity, appropriate compliance and PI monitoring at the local level with this CPG.
References
- Gomez D, Naveed A, Rezende J, Dennis BM, Kundi R, Benjamin E, Lawless R, Nguyen J, Duchesne J, Spalding C, Doris S, Van Skike C, Moore EE, Beckett A. Titratable partial aortic occlusion: Extending Zone I endovascular occlusion times. J Trauma Acute Care Surg. 2023 Aug 1;95(2S Suppl 1):S36-S40.
- Morrison JJ, Rasmussen TE. Noncompressible torso hemorrhage: a review with contemporary definitions and management strategies. Surg Clin North Am 2012;92(4):843–58, vii.
- Stannard A, Morrison JJ, Scott DJ, et al. The epidemiology of noncompressible torso hemorrhage in the wars in Iraq and Afghanistan. J Trauma Acute Care Surg 2013;74(3):830–4.
- Mattox KL, Feliciano DV. Role of external cardiac compression in truncal trauma. J Trauma 1982;22(11):934–6.
- Mattox KL, Wall MJ, Tsai P. Trauma thoracotomy: principles and techniques. In: Mattox KL, Moore EE, Feliciano DV, editors. Trauma. New York: McGraw Hill Medical; 2013. p. 461–7.
- Ledgerwood AM, Kazmers M, Lucas CE. The role of thoracic aortic occlusion for massive hemoperitoneum. J Trauma 1976;16(08):610–5.
- Burlew CC, Moore EE, Moore F a, et al. Western Trauma Association critical decisions in trauma: resuscitative thoracotomy. J Trauma Acute Care Surg 2012;73(6):1359–63. Working Group Ad Hoc Subcommittee on Outcomes, American College of Surgeons-Committee on Trauma. Practice Management Guidelines for Emergency Department Thoracotomy. J Am Coll Surg 2001;193(3):303–9.
- Seamon MJ, Fisher CA, Gaughan JP, Kulp H, Dempsey DT, Goldberg AJ. Emergency department thoracotomy: survival of the least expected. World J Surg 2008;32(4):604–12.
- Branney SW, Moore EE, Feldhaus KM, Wolfe RE. Critical analysis of two decades of experience with postinjury emergency department thoracotomy in a regional trauma center. J Trauma 1998;45(1):85–7.
- Passos EM, Engels PT, Doyle JD, et al. Societal costs of inappropriate emergency department thoracotomy. J Am Coll Surg 2012;214(1):18–25.
- Edens JW, Beekley AC, Chung KK, et al. Longterm outcomes after combat casualty emergency department thoracotomy. J Am Coll Surg 2009;209(2):188–97.
- Mitchell TA, Waldrep KB, Sams VG, et al. An 8-year review of Operation Enduring Freedom and Operation Iraqi Freedom resuscitative thoracotomies. Mil Med 2015;180(3):S33-S36.
- Joint Trauma System, Emergent Resuscitative Thoracotomy Clinical Practice Guideline, 18 Jul 2018; https://jts.health.mil/index.cfm/PI_CPGs/cpgs Accessed Mar 2020.
- Joseph B, Zeeshan M, Sakran JV, et al. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma. JAMA Surg 2019;154:500.
- Ordoñez CA, Parra MW, Manzano-Nunez R, et al. Intraoperative combination of resuscitative endovascular balloon occlusion of the aorta and a median sternotomy in hemodynamically unstable patients with penetrating chest. J Trauma Acute Care Surg. May 2018; 84(5):752–757,
- Boczar ME, Howard MA, Rivers EP, et al. A technique revisited: Hemodynamic comparison of closed- and open-chest cardiac massage during human cardiopulmonary resuscitation. Critical Care Medicine. 23(3):498-503, March 1995.
- Davidson AJ, Russo RM, Reva VA, et al. The pitfalls of REBOA: risk factors and mitigation strategies. J Trauma Acute Care Surg 2017;84:192–202.
- Teeter WA, Bradley MJ, Romagnoli A, et al. Treatment effect or effective treatment? Cardiac compression fraction and end-tidal carbon dioxide are higher in patients with resuscitative endovascular balloon occlusion of the aorta compared with resuscitative thoracotomy and open- chest cardiac massage. Am Surg. 2018 Oct 1;84(10):1691-1695.
- Moore L, Brenner M, Kozare RA, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg 2014;79:523-532.
- Abe T, Uchida M, Nagata I, et al. Resuscitative endovascular balloon occlusion of the aorta versus aortic cross clamping among patients with critical trauma: a nationwide cohort study in Japan. Crit Care 2016;20:400-410.
- Inoue J, Shiraishi A, Yoshiyuki A, Haruta K, Matsui H, Otomo Y. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: A propensity score analysis. J Trauma Acute Care Surg 2016;80:559-567.
- Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score/adjusted untreated patients. J Trauma Acute Care Surg. 2015;78:721-728.
- Perina DG, Kang CS, Bulger EM, et al. Authors' Response to Letter to the Editor by Allen et al regarding Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) by Brenner et al. Trauma Surg Acute Care Open. 2018 Mar 6;3(1):e000172.
- Manzano-Nunez R, Orlas CP, Herrera-Escobar JP, et al. A meta-analysis of the incidence of complications associated with groin access after the use of resuscitative endovascular balloon occlusion of the aorta in trauma patients. J Trauma Acute Care Surg. 2018 Sep;85(3):626-634.
- Taylor JR, Harvin JA, Martin C, Holcomb JB, Moore LJ. Vascular complications from resuscitative endovascular balloon occlusion of the aorta: life over limb? J Trauma Acute Care Surg. 2017;83(1 Suppl1):S120–S123.
- DuBose JJ1, Scalea TM, Brenner M, Skiada D, et al. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016 Sep;81(3):409-19
- Brenner M, Inaba K, Aiolfi A, DuBose J, et al. Resuscitative Endovascular Balloon Occlusion of the Aorta and Resuscitative Thoracotomy in select patients with hemorrhagic shock: early results from the American Association for the Surgery of Trauma's Aortic Occlusion in Resuscitation for Trauma and Acute Care Surgery Registry. J Am Coll Surg. 2018 May; 226(5):730-740.
- Doucet J, Coimbra R. REBOA: is it ready for prime time? J Vasc Bras 2017;16:1–3. Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg 2015;78:721–8.
- Ribeiro Junior MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg 2018;13:20.
- Matsumura Y, Matsumoto J, Kondo H, et al. Early arterial access for REBOA is related to survival outcome in trauma. J Trauma Acute Care Surg 2018;1.
- Romagnoli A, Teeter W, Pasley J, et al. Time to aortic occlusion: it’s all about access. J Trauma Acute Care Surg 2017;83:1161–4.
- Brenner M, Moore L, Teeter W, et al. Exclusive clinical experience with a lower profile device for resuscitative endovascular balloon occlusion of the aorta (REBOA). Am J Surg 2019;217:1126–9.
- Matsumura Y, Matsumoto J, Kondo H, et al. Fewer REBOA complications with smaller devices and partial occlusion: evidence from a multicentre registry in Japan. Emerg Med J 2017;34:793–9.
- Markov NP, Percival TJ, Morrison JJ, et al. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery 2013;153(6):848–56.
- Bukur M, Gorman E, DiMaggio C, Frangos S, Morrison JJ, Scalea TM, Moore LJ, Podbielski J, Inaba K, Kauvar D, Cannon JW, Seamon MJ, Spalding MC, Fox C, DuBose JJ; and the AAST AORTA Study Group. Temporal Changes in REBOA Utilization Practices are Associated With Increased Survival: an Analysis of the AORTA Registry. Shock. 2021 Jan 1;55(1):24-32.
- Maiga AW, Kundi R, Morrison JJ, Spalding C, Duchesne J, Hunt J, Nguyen J, Benjamin E, Moore EE, Lawless R, Beckett A, Russo R, Dennis BM. Systematic review to evaluate algorithms for REBOA use in trauma and identify a consensus for patient selection. Trauma Surg Acute Care Open. 2022 Dec 23;7(1):e000984. doi: 10.11/tsaco-2022-000984. PMID: 36578977; PMCID: PMC9791466.
- Polcz JE, Ronaldi AE, Madurska M, et al. Next-Generation REBOA (Resuscitative Endovascular Balloon Occlusion of the Aorta) Device Precisely Achieves Targeted Regional Optimization in a Porcine Model of Hemorrhagic Shock. J Surg Res 2022;280:1-9. DOI: 10.1016/j.jss.2022.06.007.
- Ho JW, Dawood ZS, Nguyen J, Diaz-Perez DA, Taylor ME, Chtraklin K, Jin G, Liu B, Ober RA, Alam HB. Finding the Right Balance: Partial REBOA in a Swine Model of Uncontrolled Vascular Injury. J Am Coll Surg. 2024 Jan 1;238(1):32-40. doi: 10.1097/XCS.0000000000000881. Epub 2023 Oct 23. PMID: 37870240.
- Ho JW, Jin G, Nguyen J, Keeney-Bonthrone TP, Diaz-Perez DA, Dawood ZS, Kemp MT, Alam JS, Gauger MA, Shaikh A, Chtraklin K, Liu B, Alam HB. Prolonging the zone 1 aortic occlusion time to 4 hours using a partial resuscitative endovascular balloon in a swine model. J Trauma Acute Care Surg. 2023 Aug 1;95(2S Suppl 1):S129-S136.
- Meyer CH, Beckett A, Dennis BM, Duchesne J, Kundi R, Pandya U, Lawless R, Moore E, Spalding C, Vassy WM, Nguyen J; AAST AORTA Study Group. pREBOA vs ER-REBOA™ impact on blood utilization and resuscitation requirements: A pilot analysis. J Trauma Acute Care Surg. 2025 Jan 1;98(1):87-93. doi: 10.1097/TA.0000000000004391
- Gondek S, Hamblin S, Raley J, Nguyen J, Pandya U, Duchesne J, Smith A, Moore E, Ammons LA, Beckett A, Vassy M, Carlisle P, Dennis B. A PROMPT Update on Partial REBOA: Initial Clinical Data and Overview of the DoD-Funded Partial REBOA Outcomes Multicenter ProspecTive (PROMPT) Study. Mil Med. 2024 Aug 19;189(Supplement_3):284-290.
- Morrison J, Akrish E, Kindrat V, Raley J, Carlisle P, Spalding C. Use of Partial Resuscitative Endovascular Balloon Occlusion of the Aorta by Ukrainian Medical Personnel to Sustain and Evacuate Warfighters Beyond 30 Minutes. [Podium presentation]. (26-27 Aug. 2024 Military Health System Research Symposium, Orlando, Florida.
- Glaser J, Teeter W, Fernandez N. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) as an adjunct to damage control surgery for combat trauma. JEVTM, [S.l.], v. 1, n. 1, p. 58-62, Aug. 2017.
- Manley JD, Mitchell BJ, Dubose JJ, et al. A modern case series of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in an out-of-hospital, combat casualty care setting. J Spec Oper Med 2017; 17 (1), 1-8.
- Reva VA, Hörer TM, Makhnovskiy AI. Field and en route resuscitative endovascular occlusion of the aorta: A feasible military reality? J Trauma Acute Care Surg. 2017 Jul;83(1 Suppl 1):S170-S176.
- Northern DM, Manley JD, Lyon R, et al. Recent advances in austere combat surgery: Use of aortic balloon occlusion as well as blood challenges by special operations medical forces in recent combat operations. J Trauma Acute Care Surg. 2018 Jul;85(1S Suppl 2):S98-S103
- Lyon RF, Northern DM. REBOA by a non-surgeon as an adjunct during MASCAL. Am J Emerg Med. 2018 Jun;36(6):1121.e5-1121.e6. doi: 10.1016/j.ajem.2018.02.013. Epub 2018 Feb 13.
- Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage: J Trauma Acute Care Surg 2015;78(5):1054–8.
- Van Skike CE, Baer DG, Spalding MC, Radomski M. Complete and Partial Resuscitative Endovascular Balloon Occlusion of the Aorta for Hemorrhagic Shock. J Vis Exp 2022(183). DOI: 10.3791/63767.
- Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma 2011;71(6):1869–72.
- Tibbits EM, Hoareau GL, Simon MA, et al. Location is everything: the hemodynamic effects of REBOA in zone 1 versus zone 3 of the aorta. J Trauma Acute Care Surg. 2018;85(1):101–107.
- Reva VA, Matsumura Y, Hörer T, et al. Resuscitative endovascular balloon occlusion of the aorta: what is the optimum occlusion time in an ovine model of hemorrhagic shock? Eur J Trauma Emerg Surg. 2018 Aug;44(4):511-518.
- Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017 Jan 1;80(1):6-15. doi: 10.1227/NEU.0000000000001432.
- Necsoiu C, Jordan BS, Choi JH, Moon JJ, Espinoza MD, Gremmer BJ, Batchinsky AI, Cancio LC. Mitigating Ischemia-Reperfusion Injury Using a Bilobed Partial REBOA Catheter: Controlled Lower-Body Hypotension. Shock. 2021 Mar 1;55(3):396-406. doi: 10.1097/SHK.0000000000001640. PMID: 32826820.
- Bekdache O, Paradis T, Shen YBH, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): indications: advantages and challenges of implementation in traumatic non-compressible torso hemorrhage. Trauma Surgery & Acute Care Open 2019;4:e000262.
- Kauvar DS, Dubick MA, Martin MJ. Large animal models of proximal aortic balloon occlusion in traumatic hemorrhage: review and identification of knowledge gaps relevant to expanded use. J Surg Res 2019;236:247–58.
- Villamaria CY, Eliason JL, Napolitano LM, et al. Endovascular Skills for Trauma and Resuscitative Surgery (ESTARS) course: curriculum development, content validation, and program assessment. J Trauma Acute Care Surg 2014;76(4):929–35; discussion 935–6.
- Kuckelman JP, Barron M, Moe D, et al. Extending the golden hour for zone 1 resuscitative endovascular balloon occlusion of the aorta: improved survival and reperfusion injury with intermittent versus continuous resuscitative endovascular balloon occlusion of the aorta in a porcine severe truncal hemorrhage model. J Trauma Acute Care Surg. 2018;85(2):318–326.
- Marble J, Patel NTP, Lane MR, Williams TK, Neff LP, Johnson MA. The physiology of aortic flow and pressures during partial resuscitative endovascular balloon occlusion of the aorta in a swine model of hemorrhagic shock. J Trauma Acute Care Surg. 2022 Aug 1;93(2S Suppl 1):S94-S101. doi: 10.1097/TA.0000000000003667. Epub 2022 May 12. PMID: 35545802.
- Johnson MA, Tibbits EM, Hoareau GL, et al. Endovascular perfusion augmentation for critical care: partial aortic occlusion for treatment of severe ischemia-reperfusion shock. Shock. 2019 May;51(5):659-666.
- Brenner M, Hoehn M, Pasley J, et al. Basic endovascular skills for trauma course: bridging the gap between endovascular techniques and the acute care surgeon. J Trauma Acute Care Surg 2014;77(2):286–91.
- Brenner M, Hoehn M, Stein DM, et al. Central pressurized cadaver model (CPCM) for resuscitative endovascular balloon occlusion of the aorta (REBOA) training and device testing. J Trauma Acute Care Surg 2015;78(1):197–200.
- Glaser JJ, Fisher AD, Shackelford SA, Butler F, Rasmussen TE. A contemporary report on U.S. military guidelines for the use of whole blood and resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2019 Jul;87
- Benham DA, Carr MJ, Wessels L, Lee JJ, Calvo RY, Schrader A, Holtestaul T, Lammers D, Jones I, Connor J, Weiss J, Eckert MJ, Krzyzaniak M, Martin MJ. Validation of a miniaturized handheld arterial pressure monitor for guiding full and partial REBOA use during resuscitation. Eur J Trauma Emerg Surg. 23 Oct 2022, 49(2):795-801 https://doi.org/10.1007/s00068-022-02121-8 PMID: 36273349
- Pasley JD, Teeter WA, Gamble WB, et al. Bringing Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) Closer to the Point of Injury. J Spec Oper Med. 2018 Spring;18(1):33-36.
- Saito N, Matsumoto H, Yagi T, et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg 2015;78(5):897–903; discussion 904.
- Simon MA, Tibbits EM, Hoareau GL, et al. Lower extremity cooling reduces ischemia-reperfusion injury following Zone 3 REBOA in a porcine hemorrhage model. J Trauma Acute Care Surg. 2018 Sep;85(3):512-518.
- Morrison JJ, Ross JD, Rasmussen TE, et al. Resuscitative endovascular balloon occlusion of the aorta: a gap analysis of severely injured UK combat casualties. Shock 2014;41(5):388–93.
- Johnson MA, Williams TK, Ferencz SE, et al. The effect of resuscitative endovascular balloon occlusion of the aorta, partial aortic occlusion and aggressive blood transfusion on traumatic brain injury in a swine multiple injuries model. J Trauma Acute Care Surg. 2017 Jul; 83(1):61-70.
- Williams AM, Bhatti UF, Dennahy IS, Graham NJ, Nikolian VC, Chtraklin K, Chang P, Zhou J, Biesterveld BE, Eliason J, Alam HB. Traumatic brain injury may worsen clinical outcomes after prolonged partial resuscitative endovascular balloon occlusion of the aorta in severe hemorrhagic shock model. J Trauma Acute Care Surg. 2019 Mar 1;86(3):415-23.
- Thrailkill, Marianne & Gladin, Kevin & Thorpe, Catherine & Roberts, Teryn & Choi, Jae & Chung, Kevin & Necsoiu, Corina & Rasmussen, Todd & Cancio, Leopoldo & Batchinsky, Andriy. (2021). Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA): update and insights into current practices and future directions for research and implementation. SJTREM. 29. 10.1186/s13049-020-00807-9.
- Williams AM, Bhatti UF, Dennahy IS, Graham NJ, Nikolian VC, Chtraklin K, Chang P, Zhou J, Biesterveld BE, Eliason J, Alam HB. Traumatic brain injury may worsen clinical outcomes after prolonged partial resuscitative endovascular balloon occlusion of the aorta in severe hemorrhagic shock model. J Trauma Acute Care Surg. 2019 Mar 1;86(3):415-23.
- Brenner ML, Moore LJ, Dubose JJ, et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg 2013;75(3):506–11.
- Kauvar DS, Dubick MA, Martin MJ. Large animal models of proximal aortic balloon occlusion in traumatic hemorrhage: review and identification of knowledge gaps relevant to expanded use. J Surg Res 2019;236:247–58.
- Bulger EM, Perina DG, Zaffer Q,et al. Clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA) in civilian trauma systems in the USA, 2019: a joint statement from the American College of Surgeons Committee on Trauma, the American College of Emergency Physicians, the National Association of Emergency Medical Services Physicians and the National Association of Emergency Medical Technicians. Trauma Surg Acute Care Open 2019;4:e000376. doi:10.1136/tsaco-2019-000376
- Butler FK Jr, Holcomb JB, Shackelford S, et al. Advanced resuscitative care in tactical combat casualty care: TCCC Guidelines Change 18-01:14 October 2018. J Spec Oper Med. Winter 2018;18(4):37-55.
- Brenner M, Bishoy Z, Coimbra R, AAST Multi-Institutional Trials Committee, et al. Right into the danger zone: complications of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) at zone 1 and 3 from the AAST aortic occlusion for resuscitation in trauma and acute care surgery (AORTA) trial. Presented at the 2019 AAST meeting.
- Williams T, Neff L, Johnson, MA. Letter to the Editor: Intermittent REBOA Translational Science Papers, J Trauma Acute Care Surg: Aug 28, 2019 - Volume Publish Ahead of Print - Issue – p doi: 10.1097/TA.0000000000002496
- Fisher AD, Teeter WA, Cordova CB, et al. The Role I Resuscitation Team and Resuscitative Endovascular Balloon Occlusion of the Aorta. J Spec Oper Med. Summer 2017;17(2):65-73.
- Joint Trauma System, Damage Control Resuscitation CPG, 12 Jul 2019; https://jts.health.mil/index.cfm/PI_CPGs/cpgs Accessed Mar 2020.
- Ordoñez CA, Parra MW, Caicedo Y, Rodríguez-Holguín F, García AF, Serna JJ, Serna C, Franco MJ, Salcedo A, Padilla-Londoño N, Herrera-Escobar JP, Zogg C, Orlas CP, Palacios H, Saldarriaga L, Granados M, Scalea T, McGreevy DT, Kessel B, Hörer TM, Dubose J, Brenner M; AAST-AORTA Investigators*, ABO Trauma Registry Group*. Critical systolic blood pressure threshold for endovascular aortic occlusion-A multinational analysis to determine when to place a REBOA. J Trauma Acute Care Surg. 2024 Feb 1;96(2):247-255. doi: 10.1097/TA.0000000000004160. Epub 2023 Oct 19.
Appendix A: Traumatic Arrest Algorithm
- Blunt trauma with no major chest bleeding seen on CXR, ultrasound, or bilateral chest tubes
- Penetrating trauma to abdomen/pelvis
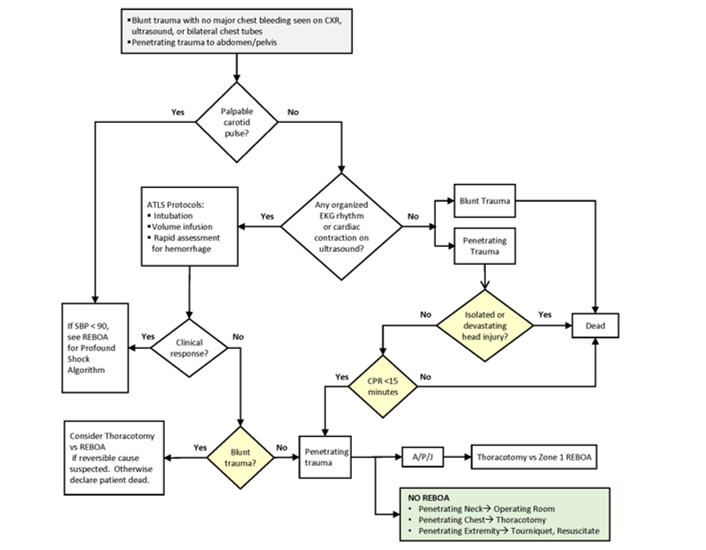
REBOA: Resuscitative Endovascular Balloon Occlusion of the Aorta; CXR: Chest X-Ray; EFAST: Extended Focused Assessment with Sonography for Trauma; ATLS: Advanced Trauma Life Support; EKG: Electrocardiogram; SBP: Systolic Blood Pressure; CPR: Cardiopulmonary Resuscitation; A/P/J: Abdomen/Pelvis/ Junctional Lower Extremity.
Zone 1 REBOA: placement of aortic balloon in the descending thoracic aorta (insert catheter to Zone 1 markers (45 - 49 cm) or measure the balloon to mid sternum, or /P-tip to the sternal notch).
Zone 3 REBOA: placement of aortic balloon directly above the aortic bifurcation (insert catheter to Zone 3 markers (26 - 29 cm) or measure the balloon to the umbilicus or P-tip to the xyphoid process).
Appendix B: Algorithm For Use of REBOA For Profound Shock
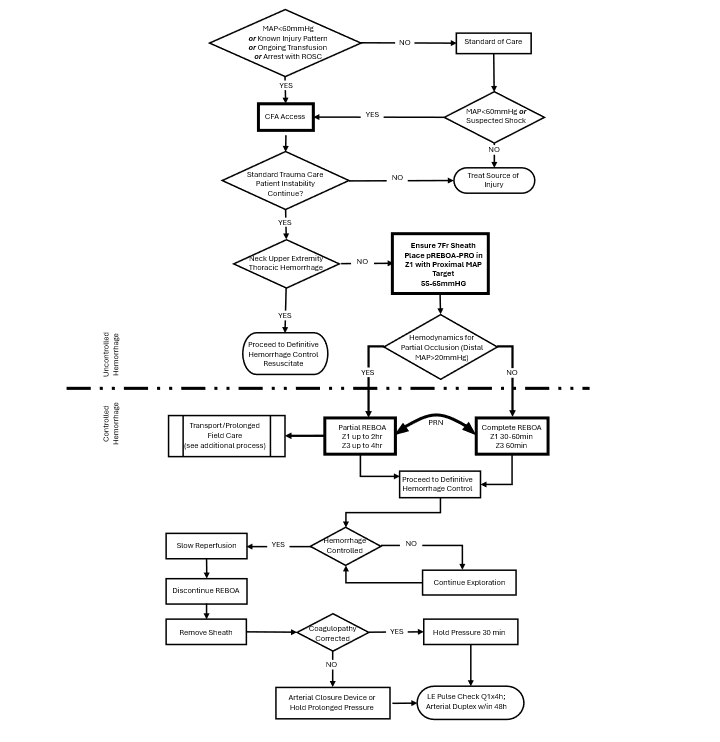
REBOA: Resuscitative Endovascular Balloon Occlusion of the Aorta; CXR: Chest X-Ray; EFAST: Extended Focused Assessment with Sonography for Trauma; ATLS: Advanced Trauma Life Support; EKG: Electrocardiogram; SBP: Systolic Blood Pressure; CPR: Cardiopulmonary Resuscitation; A/P/J: Abdomen/Pelvis/ Junctional Lower Extremity.
Zone 1 REBOA: Placement of aortic balloon in the descending thoracic aorta (insert catheter to Zone 1 markers (45 - 49 cm) or measure P-tip from the sternal notch to the arterial sheath).
Zone 3 REBOA: Placement of aortic balloon directly above the aortic bifurcation (insert catheter to Zone 3 markers (26 - 29 cm) or measure P-tip from the xyphoid process to arterial sheath).
Appendix C: REBOA STEPS USING 7 FRENCH ER-REBOA-PLUS™
STEP 1: Arterial Access and Positioning of the Sheath
Access to the arterial circulation for REBOA for trauma should be obtained through the common femoral artery using one of three techniques: percutaneous, open exposure (e.g., cut down), or exchange over a guide wire from an existing common femoral arterial line.
Ultrasound is used to identify the common femoral artery above the branch of the profunda and the needle visualized passing into the common femoral artery (linear array transducer preferred). Ultrasound guided access improves first pass access and decreases complications.1 Once identified, the artery should be entered at a 45-degree angle with the needle, using either a 5 Fr micropuncture kit or 18 gauge femoral arterial line kit. After the wire has been passed into the artery, the needle is removed and a small incision made at the interface of the wire and skin and the catheter is passed over the wire.
Using landmarks, the location of the inguinal ligament is identified between the Anterior Superior Iliac Spine and pubic symphysis (NOT the inguinal crease). The common femoral artery is then accessed 2 cm below the inguinal ligament.
Selection and Positioning of Initial Sheath:
If REBOA is indicated, the arterial access catheter must be upsized to a 7 Fr sheath. This maneuver is accomplished by placing a 0.035 guide wire greater than twice the length of the existing arterial catheter through its inner lumen allowing the catheter to be removed over the wire while maintaining arterial access. After a larger opening is created at the wire/skin interface, the 7 Fr working sheath with its internal dilator in position can be inserted over the wire. When urgently needed, a 7 Fr sheath may be placed as the initial step by placing the 7 Fr sheath over the 0.035 guide wire, though this can increase risk of access site damage.
The sheath’s internal dilator must be firmly held in place to allow a smooth reverse taper from the wire to the diameter of the sheath to avoid arterial intimal injury. Once the dilator and sheath have been advanced over the wire through the skin into the artery, the dilator and wire are removed, leaving the sheath in place. It is important that the operator assure that the stopcock is in the “off” position to reduce bleeding.
STEP 2: Selection and Positioning of the Balloon
The two products covered by this CPG are the ER REBOA-PLUS and the pREBOA-PRO™ (Prytime Medical, Boerne, TX) catheters. ER-REBOA-PLUS™ is a complete occlusion balloon and the pREBOA-PRO™ is a partial occlusion balloon. These are currently the products chosen by the DoD. These catheters are wire-free and fluoroscopy free and smaller caliber than previously used balloons, allowing fewer steps for insertion and a smaller introducer sheath (7 Fr). They also have above balloon arterial pressure monitoring capability.
Attach 30 ml syringe to the balloon port. The syringe will be filled with 30 ml of saline. Negative pressure should be applied to the balloon to remove any air, then locked in place with the plunger at the 30 ml mark on the syringe. Air should be evacuated from the syringe. If using the pREBOA-PRO™ device there is an over pressurization safety valve proximal to the white valve on the balloon port. With the white balloon port in the locked position the safety valve should be primed by pressurizing the 30 ml saline filled syringe until saline escapes the safety valve.
The a-line port of the catheter should be flushed with saline. The balloon will now pass easily into the peel-away sheath.
If using the pressure monitoring capabilities, the pressure sensor and tubing should be attached to the catheter’s arterial stopcock and flushed with saline using standard arterial line setup and transducer connected to a monitor. Once the catheter is inserted, continuous care must be taken to prevent inadvertent emboli (air, thrombus, etc.) as well as keeping the a-line patent.
For Zone 1 occlusion, the catheter should be inserted to Zone 1 markers (between 45 - 49 cm, or measured with the balloon from the midsternum, or the P-tip from the sternal notch to the femoral access catheter). For Zone 3 occlusion, the catheter should be inserted to Zone 3 markers (between 26 - 29 cm, or the balloon measured at the umbilicus or the P-tip measured from the xiphoid process to the femoral access catheter). Distances are noted on the catheter shaft.
The peel away sheath is advanced over the P-tip and balloon to protect these as they enter the 7 Fr sheath. The peel away sheath is advanced into the end of the 7 Fr sheath approximately 5mm or until it hits a “stop.” The REBOA catheter is then advanced 10cm into the sheath. The peel away sheath can then be slid back onto the catheter hub or removed, if full advancement is necessary. The catheter should be advanced to the predetermined depth. Plain film x-ray, ultrasound, or fluoroscopy can confirm correct positioning of the catheter and adjustments can be made, if necessary, prior to inflation. There are two radio-opaque markers on the catheter to designate the location of the balloon. In cases of arrest there is no role for position confirmation and this can be done at a later time when the patient is stable.
STEP 3: Inflation of the Balloon, Securing of the Apparatus, and Monitoring
A 30 ml syringe should be used. Fill syringe to 24 ml with 1/3 iodinated contrast and 2/3 saline, or all saline if contrast not available. 2 If using the ER REBOA-PLUS the balloon should be inflated until the blood pressure is augmented and contralateral femoral pulse is stopped, approximately 8 ml for Zone 1 or 2 ml for Zone 3. If using the pREBOA-PRO™ catheter balloon inflation is titrated to the patient's physiologic response.
Do not over-inflate the ER REBOA-PLUS balloon—balloon capacity is 24 ml—over-inflation can rupture the balloon or damage the aorta. The pREBOA-PRO™ safety valve is designed to protect the balloon from overinflation or too rapid inflation. Balloon inflation can be guided by fluoroscopy, hemodynamic response, and/or loss of the contralateral pulse. When fluoroscopy is available, inflate the balloon until the outer edges of the balloon change from convex to parallel as the balloon takes on the contour of the aortic wall. When inflation appears adequate to gain aortic wall apposition and/or central blood pressure is augmented, the three-way stopcock on the shaft of the balloon should be locked to maintain inflation and occlusion while other maneuvers are undertaken. Confirmatory X-ray may be used for radiographic confirmation of location. If no imaging is available in the austere environment, definitive confirmation of the balloon positioning should be accomplished directly with “hands-on” at the time of laparotomy. If the balloon is found to be malpositioned (e.g., Zone 2) the balloon can be deflated and catheter positioned to Zone 1 or 3 and the balloon re-inflated.
Securing the Inflated Balloon and Sheath:
As the central aortic pressure improves, the catheter will move caudally. To prevent catheter migration, HOLD the catheter in place or secure the catheter to the sheath, and sheath to the patient with a central line attachment device. For added monitoring and security, assign an assistant the task of holding the apparatus until balloon deflation is desired.
A trained assistant should monitor and communicate the “big three” factors imperative to maintenance of successful REBOA: MAP, maintenance of catheter position, and maintenance of occlusion (balloon inflation).
Pressure monitoring: The blood pressure should be monitored through the REBOA a-line port (above balloon pressure) and through the arterial sheath side port (the below balloon pressure). Immediately upon balloon inflation and successful arterial occlusion, the MAP increases. In order to prevent negative effects of increased circulating volume leading to hypertension, the clinician should consider partial aortic occlusion if the SBP exceeds 100 - 110 mmHg (corresponding MAP 65 mmHg). The arterial waveform should be monitored for changes including over-dampening (flattened waveform) or under-dampening (hyper-dynamic waveform). Measures should be taken to ensure that the transducer, pressure tubing, and lines are problem-free. The pressure monitoring system should include dedicated pressure tubing, fully primed and air-free, not of excessive length, and with minimal use of stopcocks. Be sure all connections are tight, but not over-tightened.
For partial occlusion, the below balloon systolic pressure should be at least 20 mmHg (corresponding MAP of 20 mmHg) and may be higher if tolerated.
Catheter position: The clinician should frequently check the measured distance of the catheter at the sheath to ensure that the catheter is not migrating. Notify the physician if catheter migration has occurred.
Maintenance of occlusion: Distal pulses should be monitored frequently. If pulses are present, and partial-REBOA is not intended, then balloon occlusion is not achieved and must be corrected. Notify the physician to add 0.5mm saline to the balloon and recheck MAP and distal pulses for evidence of complete occlusion.
STEP 4: Operative/Procedural Control of Bleeding
Control of bleeding below the diaphragm must occur very quickly, with a goal to keep the total aortic occlusion time less than 30 minutes. It is therefore important to start with damage control maneuvers to control bleeding such as clamping of the splenic or renal hilum, Pringle maneuver, clamping of any injured blood vessel, packing, or obtaining proximal and distal control of an injured blood vessel. At times, definitive control of bleeding such as solid organ removal, ligation of clamped vessels, or vascular shunt placement, may be deferred until after the REBOA has been deflated.
When partial REBOA is used, aortic occlusion is safe up to 2 hours. With pREBOA-PRO™, transition to partial occlusion as soon as the patient’s blood pressure will tolerate. Confirm partial occlusion by monitoring the below balloon pressure.
In patients with pelvic fractures, interventional radiology embolization may be considered when available, after intra-abdominal hemorrhage has been ruled out or controlled and the REBOA has been positioned in Zone 3.
STEP 5: DEFLATION OF THE BALLOON
The balloon should be deflated once hemorrhage control has been obtained. Communicating with the assistant securing the catheter and the anesthesia team is critical before deflating the balloon. When deflating the balloon turn the three-way stopcock and withdraw saline slowly as this step can be anticipated to result in significant hypotension and may result in cardiac collapse. Further resuscitation may be necessary while deflating the balloon. While one person focuses on slowly deflating the balloon, another should hold the catheter and sheath in the position to avoid unintentional migration should the need to rapidly re-inflate the balloon arise.
Complete Occlusion:
If using complete occlusion catheters (ER-REBOA-PLUS™, COBRA-OS® etc.) this step can be anticipated to result in a significant decrease in afterload and hypotension and may result in cardiac collapse. Additional resuscitation may be needed even with slow balloon deflation. The user can anticipate approximately a 10% change in flow past the balloon during deflation with as little as 0.2 ml of fluid removal. Intermittent balloon inflation and deflation may be necessary during ongoing resuscitation until hemodynamic stability is restored.
Partial Occlusion:
If using pREBOA-PRO™ (partial occlusion catheter) the user is advised to gradually remove fluid from the balloon every 10 minutes to increase the distal SBP by 20 mmHg. These small adjustments in flow over time should mitigate the ischemic reperfusion changes often encountered with removing an aortic clamp or deflating a complete occlusion aortic balloon. This slow deliberate deflation method will minimize the need for reinflation unless further hemorrhage is encountered.
STEP 6: Removal of the Balloon and Sheath
Once definitive hemorrhage control has been obtained and coagulopathy corrected, the REBOA sheath should be removed and 30 minutes of direct pressure applied to the CFA access site.
An angiogram through the sheath to document distal limb perfusion is best practice, though not always available. An aortogram may be best accomplished in the Role 3 environment with access to specialists and/or surgical backup.
The sheath should not be removed immediately prior to transport and is best removed where vascular complications can be treated and managed. If the anticipated patient transport time is less than 4 hours, the sheath may remain in place in patients with a high risk of rebleeding/continued bleeding. If patient transport time exceeds 4 hours the sheath should be removed at least 30 minutes prior to transport to allow for sufficient hemostasis at the CFA puncture site. These patients should be monitored closely en route for signs of access site complications. While the sheath is in place and up to 24hrs after removal, the patient should undergo bilateral lower extremity neurovascular checks every 1 hour. Providers should have a low threshold to involve vascular surgery or obtain a lower extremity arteriogram if any vascular change occurs.
The sheath must NEVER be left in place for transfer to a host nation hospital.
Open vascular repair may be needed if a large sheath size is used, the patient is coagulopathic, if there is technical difficulty in sheath removal, or if open femoral cutdown was used for catheter placement. If open surgical repair of the arterial access site is necessary, the femoral artery proximal and distal to the sheath entry site should be exposed to allow control. Proximally, this may require dissection for 2 cm to 3 cm underneath the inguinal ligament as an assistant uses a narrow handheld retractor (e.g., short Wylie renal vein retractor) to lift the inguinal ligament off of the femoral sheath. Exposure distal to the sheath entry site often requires identification and control of both the superficial and profunda femoris arteries.
Once proximal and distal exposure and control with vessel loops or vascular clamps have been accomplished, the sheath may be removed. Consideration should be made for passage of embolectomy catheters distally to remove any potential clot and assure back bleeding. The resulting arteriotomy, especially the intima, should be closely examined and tailored with Potts scissors if necessary to allow primary transverse closure. Closure of the arteriotomy should be performed transversely using 5-0 or 6-0 permanent monofilament suture in either an interrupted or running fashion with care to capture all layers of the arterial wall with passage of the needle. Before closing the last suture, forward bleeding and back bleeding of the arterial segments should be allowed, followed by flushing of the surface with heparinized saline. Restoration of flow through the arterial segment should be confirmed using manual palpation for pulses distally and use of continuous wave Doppler of both the artery and more distal extremity. If there is any question of flow, it is recommended to perform an angiogram and appropriate intervention if any abnormalities are noted. Closure of the soft tissues above the femoral artery is accomplished in layers using absorbable suture in the soft tissues.
- Marquis-Gravel G1, Tremblay-Gravel M1, Lévesque J1, Généreux P1,2,3, Schampaert E1, Palisaitis D1, Doucet M1, Charron T1, Terriault P1, Tessier P1. Ultrasound guidance versus anatomical landmark approach for femoral artery access in coronary angiography: A randomized controlled trial and a meta-analysis. J Interv Cardiol. 2018 Aug;31(4):496-503. doi: 10.1111/joic.12492. Epub 2018 Jan 25.
- American College of Surgeons Basic Endovascular Skills for Trauma (BEST) Course https://www.facs.org/quality-programs/trauma/education/best
Appendix D: Procedure Checklist
- Resuscitate per advanced trauma life support
- Rule out major thoracic trauma (CXR/EFAST/Bilateral chest tubes)
- Confirm abdominopelvic source of shock (Physical Exam, FAST exam and Pelvic X-ray if blunt trauma)
- Confirm bilateral femoral pulses are present
- Establish arterial access with 4 Fr (COBRA-OS®) or 7 Fr Sheath (ER-REBOA-PLUS™ or pREBOA-PRO™) in an uninjured common femoral artery
- Insert to Zone 1 or Zone 3 using the markings on the catheter
- Evacuate the balloon
- Flush the arterial line
- Insert the catheter
- Inflate the balloon
- ER-REBOA-PLUS™: Zone 1 - start with 8 ml; Zone 3 - start with 2 ml
- COBRA-OS®: Zone 1 – start with 8 ml (max 13 ml); Zone 3 – start with 2 - 3 ml
- pREBOA PRO: Inflate to above the balloon SBP 90 - 110 (MAP 55 - 65) and evaluate below the balloon pressure for adequacy of partial occlusion
- Secure the catheter
- Control major bleeding
- Monitor the patient, hemodynamics, catheter, and sheath
- Resuscitate and deflate the balloon
- Complete damage control surgery
- Remove sheath when coagulopathy corrected
- Check distal pulses after sheath removal
Appendix E: Quick Reference Guides
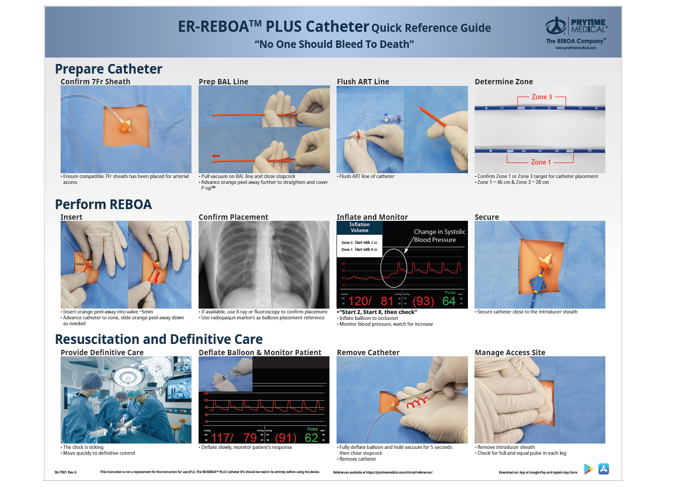
Disclaimer: The JTS does not endorse a specific catheter or products for REBOA. The purpose of this reference guide is to educate providers on the proper use of the catheter to improve clinical care.
The ER-REBOA-PLUS™ and COBRA-OS® catheter is equipped with the complete occlusion balloon, while the pREBOA-PRO™ can provide titratable partial occlusion during hemorrhagic shock.
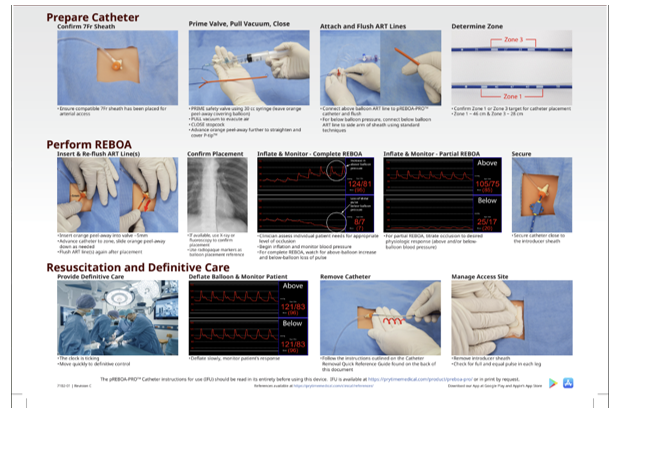
Appendix G: Class VIII Medical Materiel
- Ultrasound (linear probe for vascular access)
- Surgical set for open femoral artery exposure, including self-retaining retractors
- Scalpel (#11 or #15)
- 5 Fr micropuncture set or 18 Ga arterial line set (e.g., Cook Medical G43870; or Vascular Solutions, Inc 7208V; or Arrow Femoral Arterial Line UM-04018)
- REBOA convenience kit (Prytime Medical)
or REBOA convenience kit (Compass) (Prytime Medical PN KT1835M KIT (COMPASS) (includes two Compass devices and all items contained in the REBOA convenience kit (excluding full drape))
- Complete Occlusion - ER REBOA-Plus catheter (Prytime Medical) or COBRA-OS® (FrontLine Medical Technologies Inc.)
- Partial Occlusion - pREBOA-PRO™ catheter (Prytime Medical)
- Arterial Pressure Monitoring
- Primary Choice if Available: Standard A-line setup (x 2 if using pREBOA-PRO™)
- Secondary Choice if Arterial Line setup not available: CENTURION Compass® Universal HG-Medline PN CUHG1-INT, in-line physiologic pressure monitor (x2 if using pREBOA-PRO™)
- If able to be sterile: Ultrasound probe cover, Full body drapes, Sterile Scrub
- If not using the REBOA convenience kit (which provides all items needed for insertion):
- 7 Fr arterial sheath compatible with REBOA catheter (e.g. Cordis Avanti 402-607x) or review sheath compatibility at https://prytimemedical.com/introducer-sheath-compatibility/
- Central line securing device
- Suture and Silk ties
- 3-way stopcock
- 30 ml Luer lock syringe
- 10 ml prefilled saline syringe x3
- NON-sterile training catheters
- ER-REBOA-PLUS™ (3 non-sterile catheters + 1 access sheath)
- ER-REBOA-PLUS™ (non-sterile catheter)
- ER-REBOA PRO™ (3 non-sterile catheters + 1 access sheath)
For additional information including National Stock Number (NSN), please contact dha.ncr.med-log.list.lpr-cps@health.mil
DISCLAIMER: This is not an exhaustive list. These are items identified to be important for the care of combat casualties.
Appendix H: Telemedicine/Teleconsultation
Illustration by Raymond Samonte
Theater Patient Movement Requirements Center (TPMRC) to coordinate evacuation:
- TPMRC- Americas (NORTHCOM & SOUTHCOM), 618-817-4200
- TPMRC- East (EUCOM, AFRICOM, CENTCOM), DSN 314-480-8040
- TPMRC- West (INDOPACOM), DSN 315-448-1062
Appendix I: Information Regarding Off-Label Uses In CPGs
The purpose of this Appendix is to ensure an understanding of DoD policy and practice regarding inclusion in CPGs of “off-label” uses of U.S. Food and Drug Administration (FDA)–approved products. This applies to off-label uses with patients who are armed forces members.
Unapproved (i.e. “off-label”) uses of FDA-approved products are extremely common in American medicine and are usually not subject to any special regulations. However, under Federal law, in some circumstances, unapproved uses of approved drugs are subject to FDA regulations governing “investigational new drugs.” These circumstances include such uses as part of clinical trials, and in the military context, command required, unapproved uses. Some command requested unapproved uses may also be subject to special regulations.
Additional Information Regarding Off-Label Uses in CPGs
The inclusion in CPGs of off-label uses is not a clinical trial, nor is it a command request or requirement. Further, it does not imply that the Military Health System requires that use by DoD health care practitioners or considers it to be the “standard of care.” Rather, the inclusion in CPGs of off-label uses is to inform the clinical judgment of the responsible health care practitioner by providing information regarding potential risks and benefits of treatment alternatives. The decision is for the clinical judgment of the responsible health care practitioner within the practitioner-patient relationship.
Consistent with this purpose, CPG discussions of off-label uses specifically state that they are uses not approved by the FDA. Further, such discussions are balanced in the presentation of appropriate clinical study data, including any such data that suggest caution in the use of the product and specifically including any FDA-issued warnings.
With respect to such off-label uses, DoD procedure is to maintain a regular system of quality assurance monitoring of outcomes and known potential adverse events. For this reason, the importance of accurate clinical records is underscored.
Good clinical practice includes the provision of appropriate information to patients. Each CPG discussing an unusual off-label use will address the issue of information to patients. When practicable, consideration will be given to including in an appendix an appropriate information sheet for distribution to patients, whether before or after use of the product. Information to patients should address in plain language: a) that the use is not approved by the FDA; b) the reasons why a DoD health care practitioner would decide to use the product for this purpose; and c) the potential risks associated with such use.
Download CPG in PDF

READ FULL PDF

READ FULL PDF

READ FULL PDF

READ FULL PDF

