Vascular Injury
Joint Trauma System
Vascular Injury
SUMMARY OF CHANGES
- Contemporary epidemiology of combat vascular injuries, the impact of staged vascular care on limb salvage, and outcomes of cervical carotid injuries
- Preparation pearls for anastomosis and anastomotic techniques
- Endovascular inventory list has been updated to reflect items currently in use.
- Addition of materiel list for vascular exposure and revascularization
The treatment of vascular injuries in combat casualties can be a challenging endeavor, especially in resource constrained environments. Management of vascular trauma requires not only technical expertise on the part of the operating surgeon, but solid judgment on when to perform temporizing maneuvers versus definitive repairs. Surgeons at all Role 2 and 3 facilities need to be intimately familiar with the use of vascular shunts to stabilize a critically wounded casualty and then move them along the continuum of battlefield care. With the evolution of global conflict and risk for war with a peer enemy, a trauma system with rapid transport might not exist on a future battlefield; therefore, military surgeons at Role 2 facilities may not be able to evacuate casualties rapidly. There is also the potential for Role 3 facilities to not be readily available. Military surgeons must therefore be competent in the definitive surgical management of certain common life or limb-threatening vascular injuries. If definitive repair is required, surgeons must have the ability to ensure appropriate restoration of arterial inflow. If diagnostic capabilities are available (angiograms, plane film arteriograms), miliary surgeons need to have the skills to assess for restoration of appropriate flow. Appendix F lists the equipment required to have both a temporary vascular shunting capability and for definitive repair/reconstruction capability at any Role 2 or Role 3 facility.
EPIDEMIOLOGY OF VASCULAR INJURY
The rate of vascular injury in modern combat is five times that reported in previous wars. One in five (20%) battle injuries (non-return to duty) are classified as hemorrhage control not otherwise specified, suggesting the presence of significant bleeding.1 Using codes for specific blood vessel injuries or repairs, the rate of vascular injury is 12% in Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF), which is higher than the 1-3% reported in WWII, Korea, and Vietnam.1 Contemporary data utilizing the Department of Defense Trauma Registry reported 17.5% of combat related injuries had a vascular component.2 Extremity vessels account for 70-80% of vascular injuries while 10-15% are in the cervical region and 5-10% in the torso.1-4 Potential reasons for this increase in vascular injuries are the number of extremity amputees that have vascular trauma and also likely secondary to the widespread training and use of tourniquets on the modern battlefield, allowing casualties that would have died from extremity hemorrhage in the field to now reach medical care.5 The “golden hour” policy and the rapid medical evacuation (MEDEVAC) capability, particularly by rotary wing, that characterized OIF and OEF is also a contributing factor to patients with severe vascular injury surviving to surgical care.5
Outcome research on wartime vascular injuries show that over half of vascular injuries sustained in combat can now have an attempt at repair.1-2 This is a major shift in practice that has now been documented in more recent studies (since the last CPG update). This data confirms a complete transition away from the WWII doctrine which included a mandate against vascular injury repair and carried the recommendation to ligate all blood vessel injuries. The opportunity for military surgeons to address and repair 60% of vascular injuries in combat is a result of improved prehospital care, rapid MEDEVAC, forward positioning surgical capabilities and the use of temporary vascular shunts.2
Combat casualty data has demonstrated that the ischemic threshold for the injured extremity is half of the previously touted 6 hours.6 Preclinical data from military labs shed light on this change in dogma, and more recently published clinical studies performed in conjunction with the United Kingdom and the Joint Trauma System (JTS) have confirmed that in order to achieve functional or quality limb salvage, arterial flow must be restored in the injured limb within 3 hours, and in 1 hour or less in patients who are in hemorrhagic shock.7 To this point, the effectiveness of temporary vascular shunts in meeting the rapid restoration of perfusion goal has been confirmed in the civilian setting.9-12 These findings have substantial implications for the military in terms of training, equipping, and positioning of its surgical assets.
ROLES OF CARE AND VASCULAR INJURY
Each role of care has unique approaches to the management of vascular injury.4
Role 1 – Point of injury hemorrhage control with Tactical Combat Casualty Care principals: Pressure dressings, tourniquet placement, wound packing, etc. Initiation of evacuation and safe handoff.
Role 2 – Most surgical interventions at forward operating locations are ‘damage control’ to prioritize restoration of physiology over anatomy. Abbreviated (<1 hour) operations should focus on restoration of patient physiology, restoration of vascular flow, and focused on life and limb saving procedures. Early intervention on extremity vascular injuries is important and may make the difference in meaningful limb salvage. While physiology restoration is always the first stage of managing vascular injury +/- polytrauma, the future operating environment might require Role 2 surgical teams to embark on subsequent definitive care depending on the deployed trauma system.
- Primary amputation or ligation is also an acceptable damage control technique when other life-threatening injuries are present, or the patient is in extremis.
- If limb salvage is attempted, initiation of basic maneuvers including removal of tourniquet, exploration and control of the vascular injury, removal of clot (thrombectomy) and administration of heparinized saline through the inflow and outflow vessels are recommended. Venous injuries should be repaired after arterial inflow is restored. Data from combat casualties demonstrates high limb salvage with venous repair.
- Restoration of flow is most expeditiously established using a temporary vascular shunt, which expedites reperfusion and lowers the rates of amputation. This followed by having a low index of suspicion to perform a fasciotomy and then initiation of medical evacuation to a higher level of care (if possible) to optimize limb salvage.
- Temporary shunt placement for the initial management of proximal extremity vascular injury is associated with very high rates of successful limb salvage, and consistent shunt patency has been demonstrated for periods up to 12 hours.9,10,11 However, experience with shunt utilization without systemic anticoagulation for more prolonged periods is limited, and the risk of shunt thrombosis is markedly increased when used beyond 12 hours.8,12
- If a significant delay before definitive vascular management is anticipated, Role 2 surgeons should consider definitive management of the injury with repair/reconstruction (if appropriate equipment available, see Appendix F) or ligation, depending on the patient’s stability and the experience level of the surgeon. An option for more long-term temporary ‘shunting’ would be using a Gortex™ or Dacron ™ graft if available. This is a more ‘stable shunt’ and does not risk using vein when good autologous repair might be limited. Wartime arterial injury usually necessitates staged care; recent data demonstrates similar rates of limb salvage between staged interventions at Role 2 and initial management at Role 3.13
- When MEDEVAC to a Role 3 is readily and rapidly available, Role 2 surgeons should NOT perform definitive vascular repair/reconstruction as this may risk early graft failure during en-route care and may risk limb salvage opportunities and long-term functional outcomes.
- During evacuation, shunts should be backed up with tourniquets in the event they become dislodged during transport.
- Role 3 – In most circumstances, if clinically indicated, definitive vascular repair should be accomplished at the Role 3. Autologous vein, usually saphenous, is used to repair the injured artery. Venous repair should be attempted if the casualty’s physiology is amenable to a longer operation.
- Synthetic polytetrafluoroethylene (PTFE) conduit can be used in the absence of appropriate autologous vein, with appropriate soft tissue coverage and antibiotic administration.
- During aeromedical evacuation to the Role 4 (usually fixed-wing), the extremity will be difficult to examine, therefore Role 3 surgeons must assure adequacy of limb perfusion prior to transfer. There should be a low threshold to perform a fasciotomy if there is any question about compartment syndrome or an increased risk of compartment syndrome during transfer to Role 4.
- Penetrating wartime vascular injuries are almost universally associated with severe and contaminated soft tissue injuries. These wounds should be debrided daily in the initial phase of care and prior to transfer.
- Primary amputation or ligation is also an acceptable damage control technique at this echelon of care when other life-threatening injuries are present, or the patient is in extremis.
- Outside Continental U.S. (OCONUS) Role 4 – Assessment of vascular repair including repeated evaluation (OR or bedside) of soft tissue wounds and adequacy of tissue coverage. Contaminated or extensive soft tissue injuries should go to the OR every day until there is no further evidence of myonecrosis or gross contamination. Wounds and vascular patency should be assessed within 24 hours prior to a long fixed-wing flight. Consideration should be made for replacement of prosthetic bypass material with autologous vein conduit.
- Continental U.S. (CONUS) Role 4– Surveillance of vascular repair with duplex or computed tomography-angiography (CTA) as well as assessment of soft tissue wounds and adequacy of tissue coverage is performed at this echelon.14 Angiography (computed tomography or conventional) has particular utility in the identification of more subtle vascular injuries (e.g., traumatic pseudoaneurysm, arteriovenous fistula) following blast injury.15 In some instances, revision of at-risk repairs is necessary when bypasses are identified as having a stenosis or inadequate tissue coverage leaving them prone to infection and blowout.16 Finally, delayed revascularization of viable but poorly perfused extremities, i.e. when ligation was performed as the initial method of management can be accomplished.
DIAGNOSIS OF VASCULAR INJURY
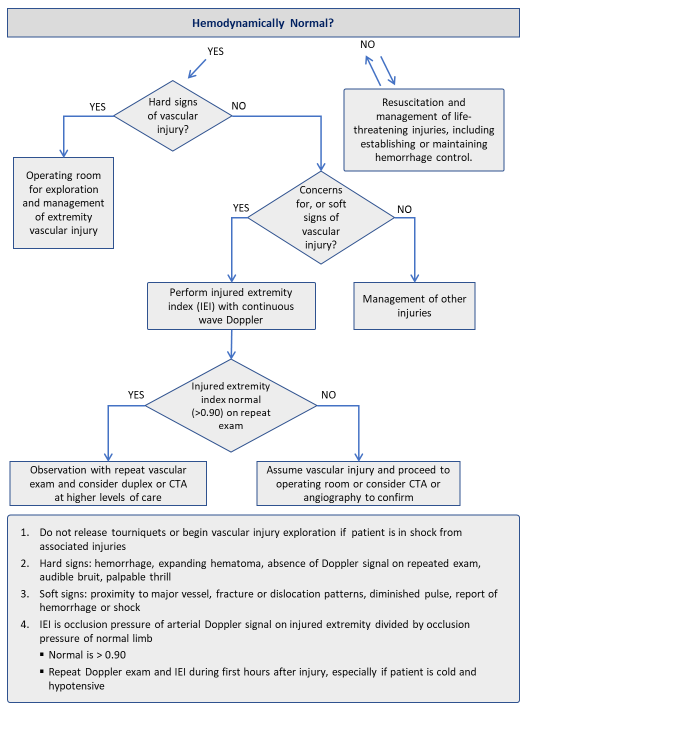
Hard signs such as active hemorrhage, absence of distal pulse, palpable thrill or expanding hematoma require immediate management in the operating room, generally with exploration of the injury site with wide exposure to enable vascular control. Ischemia in this situation is defined as the absence of Doppler signal in the extremity on multiple attempts, including after initiation of resuscitation and warming, and initial fracture stabilization. When hard signs of injury are present, there is limited need for other diagnostic tests (i.e. CTA or angiography) which take extra time and may provide findings which cloud decision making.4,17,18 Massive transfusion should be activated if the patient is in hemorrhagic shock.
Soft signs such as history of significant hemorrhage, injury proximity to major vessels (fracture pattern, dislocation, penetrating wound, or blast injury), bruising or hematoma or question regarding the presence or absence of a palpable pulse require another diagnostic test. This additional test is commonly the continuous wave Doppler with calculation of the injured extremity index for traumatized limbs if possible and CTA or angiography for questionable torso and/or extremity vascular injuries where available. A commonly used injured extremity index for further testing is 0.9 or less for this group of patients. (See Appendix A).
The injured extremity index (IEI) is similar to the ankle-brachial index and is calculated using a manual blood pressure cuff and a continuous wave Doppler. When doppler is available, the injured extremity index should be measured in patients with:
- Soft signs of vascular injury on an injured extremity
- Diagnosis or high suspicion of posterior knee dislocation
The first step is to determine the pressure at which the arterial Doppler signal returns in the injured extremity as the cuff is deflated. This is the numerator in the equation. Next the cuff and Doppler are moved to an uninjured extremity and the pressure at which the arterial Doppler signal returns as the cuff is deflated is recorded as the denominator in the ratio. An injured extremity index greater than 0.90 is normal and has a high specificity for excluding major extremity vascular injury.19 An injured extremity index less than 0.90 is abnormal, and further diagnostic testing as described below, or surgical exploration should be considered.
A thorough neurovascular exam of the injured extremity should also be performed. For this exam, the injured extremity’s pulses as well as gross motor and sensory function are evaluated. The neurovascular exam findings should be performed and documented in the patient’s records. For patients with presentation or injury pattern concerning for extremity vascular injury (e.g., patients with soft signs of vascular injury but >0.9 IEI), a neurovascular exam should be performed and documented hourly for the first 24 hours and can then be expanded to every 4 hours if there are no concerning changes to the exam. This is important as deterioration of the neurovascular exam indicates high likelihood of vascular injury that may require prompt operative intervention.
Angiography has limited utility in the diagnosis of wartime extremity vascular injury mostly because the lack of availability and quality of imaging technology in austere environments. Additionally, extremity vasoconstriction associated with shock and hypothermia in the young, injured patient may lead to confusing or false positive findings on angiography. Digital Subtraction Angiography (DSA) is very useful in the setting of multiple penetrating wounds at various levels of the same extremity to determine the location and extent of injury/injuries. It is possible to do plane film angiography via an ipsilateral cut down on the femoral artery and injecting contrast through a 19–21 gauge butterfly needle and taking an image immediately after injection. DSA remains the goal standard to assess for vascular injury. In the absence of DSA, or other vascular imagining, exploration should be performed to ligate, shunt or repair the vascular injury.
Computed Tomography-Angiography (CTA) is increasingly available in a mature theater of war and has its greatest utility in the diagnosis and triage of torso and neck wounds. CTA should be used as an adjunct for extremity evaluation, as its full utility has yet to be determined and may have limited diagnostic accuracy in IED blast injuries due to metallic streak artifact. Specifically, CTA for head and neck wounds demonstrates a sensitivity of 80%.18,19 Furthermore, this modality takes additional time, proper IV contrast timing, and technical experience to provide accurate and meaningful images.
GENERAL PRINCIPLES OF VASCULAR INJURY MANAGEMENT
NOTE: See Appendix A, Appendix B and Appendix C for management based on anatomic location and Appendix F for basic equipment list
Most deploying non-vascular or non-cardiothoracic surgeons will have limited recent experience in vascular surgery. Prior to deployment, all military surgeons should take the ASSET+ course (DoD developed course currently part of the Emergency War Surgery Course). ASSET+ training was developed by military surgeons for military surgeons to given them iterative training on vascular exposures. Training for surgeons should emphasize the basic principles of vascular trauma management, including adequate exposure, proximal and distal control, vessel debridement to viable tissue, the creation of a tension-free anastomosis, repair or shunt, and adequate coverage with viable tissue. The most challenging aspect in the management of a wartime vascular injury is generally related to vascular exposure. As most of these injuries involve previously normal blood vessels, vessel suturing, and shunt placement are usually a relatively straightforward technical exercise. However, in the face of tissue destruction, hematoma, distorted anatomic landmarks, and the potential absence of a palpable pulse, the identification and adequate exposure of a wartime vascular injury can be a challenge for even an experienced surgeon. While the deploying surgeon will find additional detail regarding techniques and “pearls” in the appendices extremely valuable for pre-deployment review and as a reference during deployment, surgeons should also maximize opportunities to review anatomic exposures in cadaveric, simulation, and video settings prior to deployment. Furthermore, an atlas that covers vascular surgery and exposures should be at the immediate ready for every surgeon on a combat deployment.
- Acceptable damage control maneuver especially for small, more distal arteries and veins.
- Temporary vascular shunting to restore perfusion should be considered before ligation.
- Continuous wave Doppler should be checked before ligation to judge collateral perfusion/viability.
- Ligation of vascular injuries was the mainstay of treatment for centuries and should not be overlooked as a damage control option, especially at Role 2 facilities where operations are best abbreviated (≤ 1 hr). This technique is especially useful in small distal vessels (tibial, forearm and arm below the take-off of the profunda brachial artery) when patients are in extremis. Use of temporary vascular shunts or even repair should be considered before ligation; however, if not available or feasible, ligation should be completed. Continuous wave Doppler may also be useful in assessing perfusion to the extremity distal to the vessel in question. If there is loss of an arterial bypass during the operation or shortly thereafter with associated venous ligation, consideration should be made for venous reconstruction as the arterial bypass may be lost due to reduced extremity outflow.
Fogarty catheters are a key tool in the armamentarium of vascular injury management. Used primarily to remove thrombus, they can also be used to arrest bleeding from within the lumen of the vessel. The most common size used in extremity vascular injury is 2 and 3 Fr. At least one pass of a Fogarty should precede extremity vascular injury repair to assure removal of the traumatic thrombus burden before restoring inflow and outflow. The key tenet is not to cause native vessel damage. To lessen the risk of damage, avoid advancing the catheter too distal in the smaller vessels of the leg and arm and avoid over aggressive, static balloon inflation (i.e. angioplasty or “intimectomy”).
- Sized at 2-7 Fr; Commonly use 2 or 3 Fr catheters. Maximum balloon diameter of the 2 and 3 Fr catheters is 4 and 5 mm.
- Inflate with saline using 1cc tuberculin syringe (max inflation listed on the Fogarty hub) while withdrawing from vessel.
- Goal is clot, not intima, removal so don’t over-inflate or “drag” too much.
- A valuable tool in assessing the distance of the catheter placement and avoiding distal intimal injury is to measure externally on the skin the distance the catheter should pass.
- May be used to control bleeding from within the vessel lumen. Requires a 3-way stop cock to maintain inflation once the bleeding has been stopped.
- Inline shunts rest in the vessel (“in-situ”) while long external shunts are designed to loop.
- In-line Argyle shunts come in a cylinder container with 8, 10, 12 and 14 Fr sizes.
- In-line Javid shunts are longer and individually packaged with a ribbed edge to help keep secure.
- Sundt shunts are designed with short (15cm; inline) and long (30cm; external) profiles with a ribbed edge to help keep secure.
- Equal success has been had with Argyle, Javid and Sundt without systemic anticoagulation.
- Proximal and Distal embolectomy should be performed prior to shunt placement.
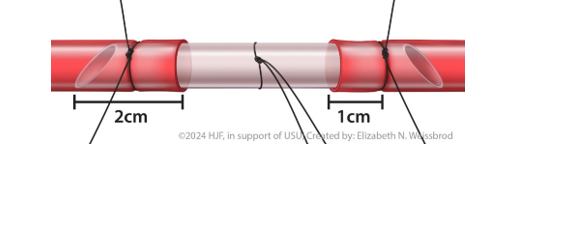
- When cutting the shunt to size, there should be at least 4cm longer than the gap between the injured ends of the vessel. For example, if there is a 3cm gap, the shunt should be approximately 7cm long with 2cm of the shunt in both the proximal and distal injured vessel. (Figure 1). This is to allow 2cm of the shunt to be in the proximal and distal ends of the artery.
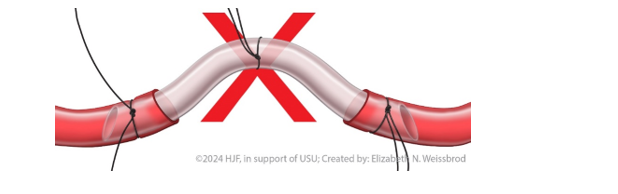
- Do not kink the shunt, as this will create turbulent flow and increase the risk of shunt thrombosis. (Figure 2)
- When placing the shunt, be careful not to injure the intima (particularly if the shunt was cut to size) as this can result in early shunt failure and necessitate a more complex definitive repair/reconstruction.
- Secured with silk ligatures no more than 1cm from each damaged end of the vessel; edges will be debrided to health tissue beyond the ligature prior to definitive repair. (Figure 1)
- If not secured well, shunts will have a tendency to migrate distally and even become dislodged. To prevent this from occurring:
- All ‘tails’ are used (6 in total).
- Vessel to vessel - not tight but to stabilize the two ends of the vessel to prevent further retraction.
- Shunt to vessel on the proximal end.
- Shunt to vessel distally.
- Do not leave these silk tail long or there is a risk of an en-route care provide ‘pulling up on them’ should there be a concern for bleeding. Cut all the tails and temporarily close the wound.
- Ensure there is a way for en-route care providers to attain proximal control if there is massive bleeding (from shunt dislodgement) during transport. This can be done with a proximal (pre-positioned) tourniquet or with vessel loops that have been secured appropriately.
- Patent for up to 6 hours; reports of longer duration exist.
- Consider shunting of concomitant vein injuries if possible.
- Shunts should be removed with formal repair in-theater prior to MEDEVAC to Role 4.
- Temporary vascular shunts are effective and should be considered in the management of nearly all extremity vascular injury patterns including proximal venous injuries. Their main advantage is provision of early restoration of flow and mitigation of the damaging effects of arterial ischemia and venous hypertension. As an abbreviated procedure compared to formal vascular repair, shunting extends the window of opportunity for limb salvage in some patterns of vascular injury. Although the patency at 3-4 hours is higher in larger, more proximal vessels (axillary/brachial and femoral/popliteal), shunts have been used effectively in smaller (distal brachial/forearm and tibial) vessels. Outcomes of extremity vascular injury managed with temporary shunts have been recorded demonstrating no adverse effect of this technique and a limb salvage advantage in the most severely injured limbs (MESS ≥ 8). 3,4,8,20
HARVESTING & USE OF AUTOLOGOUS VEIN
Pearls
- Use reversed greater saphenous vein from uninjured extremity.
- Expose at saphenofemoral junction or anterior to medial malleolus (consistent locations).
- Be sure to mark anatomically distal end as “in-flow” assuring reversal of vein conduit. This is usually done with an ‘olive tipped’ vein cannulator that is secured in the distal end of the vein.
- Introduce 18 gauge plastic vein cannula (angiocatheter) or metallic olive tip cannula to distend the vein with heparin saline.
- Because of its versatility, resistance to infection, propensity for tissue incorporation and favorable patency rates, saphenous vein for interposition graft or patch material is favored. The greater saphenous veins may be consistently located at the saphenofemoral junction (2cm medial to the pubic tubercle) or 1-2 cm anterior to the medial malleolus. Identifying the actual saphenofemoral junction is important to confirm that the vein being exposed is truly the main channel saphenous and not an accessory branch or anterior saphenous (i.e. must follow back to main saphenofemoral junction). In the setting of trauma, the vein frequently appears in-situ as “too small” or “not adequate” due to vasoconstriction or spasm.
- Nonetheless, after confirming that the vein being exposed is the main channel saphenous, the specimen should be removed and dilated on the back table with firm infusion of heparin saline using a 14–18-gauge plastic vein cannula or the metallic olive tip cannula. Persistence and this maneuver almost always result in a markedly improved and dilated vein ready to be used for repair. Reversal of the vein must also be confirmed as venous valves will not permit flow in a retrograde fashion. Smaller sutures (7-0 prolene) are often necessary if side branches need more control than that afforded by initial harvest.
USE OF PROSTHETIC GRAFT MATERIAL
- ePTFE (Gortex) or Dacron used for central torso vascular injuries (aorta, great vessels).
- Prosthetic conduit acceptable as last resort in extremities when there are no shunts, the vein is being preserved for definitive care at a later time, or the vein cannot be harvested.
- If prosthetic used in extremity injury, notify higher levels of care to facilitate surveillance.
- Prosthetic graft materials such as ePTFE (Gortex) or Dacron should be reserved for open reconstruction of the aorta and large torso vessels and used very rarely as conduit for extremity vascular injury. Wartime experience has demonstrated poor incorporation of prosthetic grafts in extremity wounds and a propensity for infection compared to saphenous vein. Additionally, extrapolation of civilian data suggests improved patency of vascular reconstructions using saphenous vein. In the rare instance (i.e. damage control) when prosthetic conduit is used for extremity vascular injury, communication with higher levels of care should occur so that appropriate surveillance or even removal of the graft and replacement with vein can occur. 3
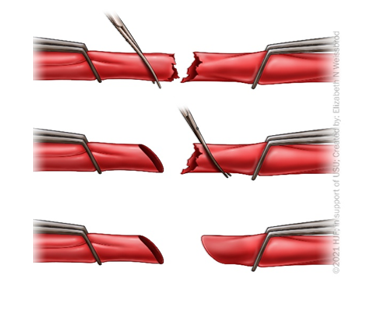
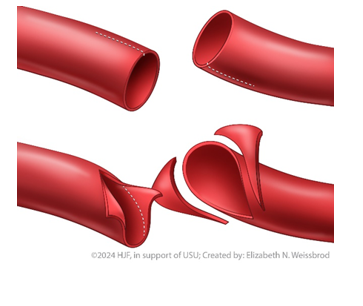
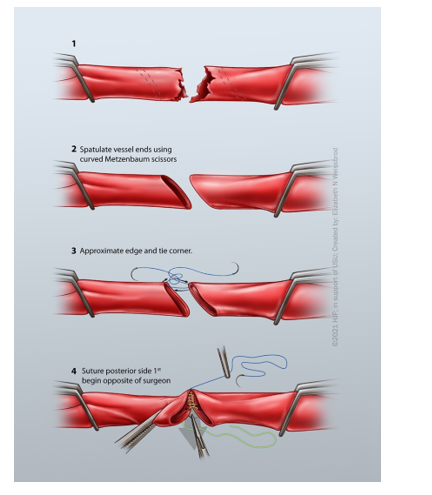
Spatulate or Bevel the Conduit
Purpose: to prevent stenosis and mitigate size mismatch between conduit and in situ vessel
Bevel: to cut the edge of the conduit and in situ vessel (Figure 4)
Spatulate: to open the vessel to increase diameter (Figure 5)
ANASTOMOSIS TECHNIQUE: PARACHUTE VS TWO-POINT VS SINGLE-POINT FIXATION
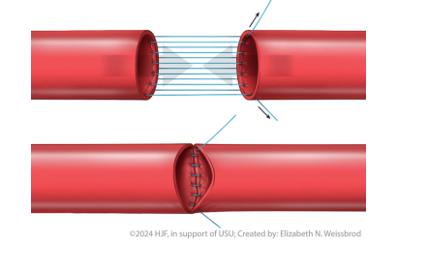
Parachute
At the beginning of the anastomosis, no knot is started (Figure 6). Sutures are placed in a running fashion until the conduit is “parachuted down.” A knot is tied at the end of the anastomosis. This technique is helpful when sewing down to something with difficult exposure (pelvis), although some surgeons prefer this technique in every case.
Two-Point Fixation
The anastomosis is started on the bottom, in which a knot is tied. A second suture is placed on the opposite side of the first knot (Figure 7). This can either be tied down or tagged to assist with offloading tension. Sutures may be placed in a running fashion from either knot and met in the middle (Figures 7 and 8). This technique is helpful when sewing vessels that are under some tension, but some surgeons always prefer this method.
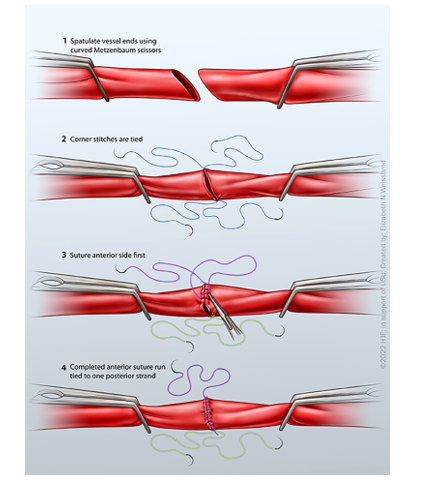
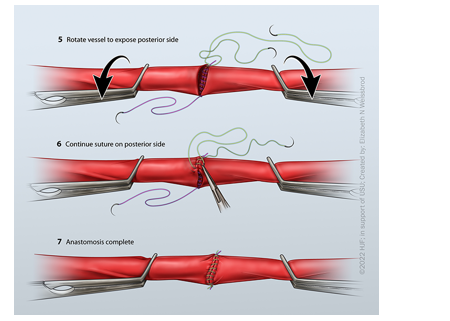
Single-Point Fixation
Similar to the two-point fixation, but a single knot is placed on the bottom of the vessels (Figure 9). The anastomosis is then carried around each side until the end, in which another knot is tied down to complete the anastomosis.
SOFT TISSUE COVERAGE & ANASTAMOTIC DISRUPTION
- Cover vascular repairs with available viable local tissue (muscle and soft tissue pedicles).
- If no soft tissue to cover, route grafts out of zone of injury (extra-anatomic).
- A poorly covered vascular anastomosis or an anastomosis in an infected wound bed, can “blowout,” but not in the early (< 5 day) period.
- Avoid direct placement of negative pressure wound therapy sponge on vascular structures.
- Soft tissue coverage of vascular repairs is required to assure incorporation and prevent infection and blowout. Option 1 is to immediately cover the repair with viable local soft tissue (muscle and adipose). If available, the negative pressure wound therapy device (VAC) is useful on top of such coverage as it provides a closed dressing which removes wound effluent and decreases bacterial counts. This wound adjunct has been found to assist with accomplishing delayed primary closure of soft tissue wounds over vascular repairs or coverage with skin grafts. The reticulated open-cell foam sponge of the VAC should not be placed directly on vessels; however, when used over viable tissue covering the vascular repair, VAC has resulted in excellent outcomes with no increase in graft-related complications or blowouts.1 If there is no viable tissue option to cover the repair, the white foam vac dressing may be used to cover the vessel, but NEVER the black foam dressing.
- If no tissue is available to cover the vascular repair, one can route an interposition graft out of the zone of injury through another myocutaneous or even subcutaneous path (extra-anatomic). As a last resort, the vascular reconstruction can be left with marginal coverage at Role 2 and 3 facilities; however, in these cases close examination must occur at Role 4 facilities. In these rare instances higher levels of care should pursue transfer of viable tissue from other locations (sartorius, rectus abdominis or other muscle) to definitively cover the repair within 5-7 days. Although it is acceptable for Role 2 and 3 providers to leave a graft with uncertain coverage, the onus of care then falls heavily on Role 4 facilities to inspect, cover, re-route or even ligate the graft to reduce the risk of catastrophic blowout. When soft tissue coverage is not possible, consideration should be made for constant proximal tourniquet placement in the event of blowout and application by nursing or evacuation assets.
- It is important to recognize that even in the best of civilian and wartime circumstances that there has been historically and remains currently a finite risk of anastomotic disruption. Using the management strategies described above the risk of graft blowout has been within an acceptably low range of 1-2% throughout the wars in Iraq and Afghanistan.19,21
- Formal control (DeBakey clamps) is acceptable but may be difficult or not advisable as it risks causing injury or may not be needed if injury is limited to the side wall of the vein.
- Initial control can be accomplished by one or more fingers on the bleeding segment.
- Organize the operating room and confirm availability of blood and central venous access.
- Central venous access above the heart if operating on injury to the inferior vena cava. If operating on an injury in the chest, central venous access is ideally at the femoral vein.
- Optimal lighting, exposure (i.e. extend incisions) and two or more suction devices.
- Avoid too small of a needle and suture which are difficult to maneuver in blood. 4-0 Prolene on an SH tapered needle is substantive suture on a needle large enough to see.
- Fingers replaced with a low profile tampanode device such as a small sponge stick or Wecksorb “K” dissector (i.e. Kitner device) as bleeding is evacuated.
- Passes of suture are made capturing muscle or soft tissue if possible (i.e. pledget-effect). Tie the knot to begin running venorrhaphy or place second pass in “figure-of-8” fashion.
- Felt pledgets can be used but may not be available.
- Hemorrhage control with ligation is preferable to patency with death from exsanguination.
ANTIPLATELET THERAPY & SYSTEMIC ANTICOAGULATION
- Utilizing regional or systemic anticoagulation will be on a case-by-case basis. Systemic anticoagulation is the ideal method, but may not be possible with high-risk concomitant injuries such as traumatic brain injury, non-operative blunt solid organ injury, etc. If contraindications for systemic anticoagulation exist, regional anticoagulation is advised.
- Antiplatelet therapy with aspirin is recommended post-operatively for arterial repairs, interposition grafts (autologous or prosthetic) and bypass grafts (autologous or prosthetic) unless concomitant injuries stated above contraindicate its use.
- Systemic anticoagulation is achieved with 50 u/kg of IV heparin with 1000 u repeated at 1 hr; repeat doses are not recommended given the propensity for bleeding in wartime injury.
- “Regional anticoagulation” is the use of heparin saline flush in the inflow/outflow vessels.
- Heparin saline is typically 10000u/liter (10U Heparin/1cc of Saline), although other mixtures with or without papaverine are acceptable; there is no evidence that other ‘vein solutions’ offer any advantage.
- The use of recombinant factor VII is no longer recommended.
PERFORMANCE IMPROVEMENT (PI) MONITORING
- All patients with penetrating injury to an extremity proximal to knee/elbow with AIS ≥ 2 or diagnosis of posterior knee dislocation.
- All patients diagnosed with injury to major artery or vein (subclavian, axillary, carotid, brachial, common femoral, superficial femoral, popliteal, common iliac, external iliac, internal iliac, aorta, vena cava, portal vein, hepatic artery, mesenteric artery, renal artery).
- All patients with penetrating injury to an extremity proximal to knee/elbow with AIS ≥ 2 or diagnosis of posterior knee dislocation have injured extremity index documented.
- All patients with penetrating injury to an extremity proximal to knee/elbow with AIS ≥ 2 or diagnosis of posterior knee dislocation have neuro and vascular exam documented.
- All patients diagnosed with injury to major artery or vein undergo revascularization (shunt or repair) or ligation at the first surgical capability (or valid explanation for delay documented) prior to transfer to next level of care.
- All patients diagnosed with injury to major artery or vein who undergo reperfusion (shunt or repair) have the procedure within 4 hours of injury.
- All Patients with major vascular injury presenting with signs of hemorrhagic shock (SBP <100, HR >100) get blood products or MTP (massive transfusion protocol) activation.
PERFORMANCE / ADHERENCE METRICS
- Number and percentage of patients in the population of interest who have injured extremity index documented.
- Number and percentage of patients in the population of interest who have neuro and vascular exam documented.
- Number and percentage of patients in population of interest who undergo revascularization (shunt, repair, ligation) prior to transfer to next level of care.
- Number and percentage of patients in population of interest whose injury undergo reperfusion (shunt or repair) or ligation within 4 hours of injury.
- Number and percentage of patients in population of interest who undergo definitive Role 2 repair/reconstruction when temporary vascular shunt was feasible, and Role 3 MEDEVAC was readily available.
- Number and percentage of patients with major vascular injury presenting with signs of hemorrhagic shock (SBP <100, HR >100) get blood products or MTP (massive transfusion protocol) activation.
- Patient Record
- Department of Defense Trauma Registry
The above constitutes the minimum criteria for PI monitoring of this CPG. System reporting will be performed annually; additional PI monitoring and system reporting may be performed as needed.
The system review and data analysis will be performed by the JTS Chief and the JTS PI team.
It is the trauma team leader’s responsibility to ensure familiarity, appropriate compliance, and PI monitoring at the local echelon with this CPG.
REFERENCES
- White JM, Stannard A, Burkhard GE, Eastridge BJ, Blackbourne LH, Rasmussen TE. The epidemiology of vascular injury in the wars in Iraq and Afghanistan. Ann Surg 2011;253:1184-9.
- Patel JA, White JM, White PW, Rich NM, Rasmussen TE. A contemporary, 7-year analysis of vascular injury from the war in Afghanistan. J Vasc Surg. 2018 Dec;68(6):1872-1879.
- Clouse WD, Rasmussen TE, Peck MA, et al. Current in theater management of wartime vascular injury: a report from Operation Iraqi Freedom. J Am Coll Surg. 2007; 204(4):625-632.
- Rasmussen TE, Clouse WD, Jenkins DH, et al. Echelons of care and the management of wartime vascular injury: A report from the 332nd EMDG/ Air Force Theater Hospital Balad Air Base Iraq. Persp Vasc Endovasc Surg. 2006;18(2):91-99.
- Isaacson, B. M., Swanson, T. M., Potter, B. K., & Pasquina, P. F. (2014). Tourniquet use in combat-injured service members: a link with heterotopic ossification? Orthopedic Research and Reviews, 6, 27–31.
- Perkins ZB, Kersey AJ, White JM, Lauria AL, Propper BW, Tai NRM, Rasmussen TE. Impact of Ischemia Duration on Lower Limb Salvage in Combat Casualties. Ann Surg. 2022 Sep 1;276(3):532-538.
- Hancock HM, Stannard A, Burkhardt GE, Williams K, Dixon P, Cowart J, Spencer J, Rasmussen TE. Hemorrhagic shock worsens neuromuscular recovery in a porcine model of hind limb vascular injury and ischemia-reperfusion. J Vasc Surg. 2011 Apr;53(4):1052-62.
- Wing W, Ji W, Wu X, Li J. Prolonged indwelling time of temporary vascular shunts is associated with increased endothelial injury in the porcine mesenteric artery. J Trauma. 2011;70:1464-70.
- Polcz JE, White JM, Ronaldi AE, et al. Temporary intravascular shunt use improves early limb salvage after extremity vascular injury. J Vasc Surg. 2021;73(4):1304-1313.
- Taller JT, Kandar JP, Greene JA, et al. Temporary vascular shunts as initial treatment of proximal extremity vascular injuries during combat operations: the new standard of care at echelon II facilities? J Trauma 2008;65:595-603
- Rasmussen TE, Clouse WD, Jenkins DH, Peck MA, Eliason JL, Smith DL. The use of temporary vascular shunts as a damage control adjunct in the management of wartime vascular injury. J Trauma. 2006; 61(1):15-21.
- Granchi T, Schmittling Z, Vasquez J Jr, et al. Prolonged use of intraluminal shunts without systemic anticoagulation. Am J Surg. 2000;180:493-497.
- Kauvar DS, Propper BW, Arthurs ZM, Causey MW, Walters TJ. Impact of Staged Vascular Management on Limb Outcomes in Wartime Femoropopliteal Arterial Injury. Ann Vasc Surg. 2020;62:119-127.
- White PW, Gillespie DL, Feuerstein IM, et al. Sixty-four slice multidetector computed tomographic angiography in the evaluation of vascular trauma. J Trauma 2010; 68: 96-102.
- Johnson ON, Fox CJ, O’Donnell S, et al. Arteriography in the delayed evaluation of wartime extremity injuries. Vasc Endo Surg 2007; 41(3): 217-224.
- Greer LT, Patel B, Via KC, Bowman JN, Weber MA, Fox CJ. Management of secondary hemorrhage from early graft failure in military extremity wounds. J Trauma 2012; 73(4): 818-824.
- Fox CJ, Starnes MW. Vascular surgery in the modern battlefield. Surg Clin N Am. 2007; 87:1193-1211.
- Lavenson GS Jr, Rich NM, Strandness DE Jr. Ultrasonic flow detector value in combat vascular injuries. Arch Surg. 1971; 103:644-647.
- Johansen K, Lynch K, Paun M, Copass M. Non-invasive vascular tests reliably exclude occult arterial trauma in injured extremities. J Trauma 1991; 31(4): 515-519.
- Gifford SM, Aidinian G, Clouse WD, et al. Effect of temporary vascular shunting on extremity vascular injury: an outcome analysis from the GWOT vascular initiative. J Vasc Surg. 2009; 50(3):549-55.
- Leininger BE, Rasmussen TE, Smith DL, Jenkins DH, Coppola C. Experience with wound VAC and delayed primary closure of contaminated soft tissue injuries in Iraq. J Trauma. 2006; 61:1207-1211.
APPENDIX A: EXTREMITY VASCULAR CARE BY ANATOMIC LOCATION
- Recommendations: Repair
- Utility of temporary shunt: Low (excellent distal collateral flow)
- Method/Conduit: Interposition graft /6-8mm ePTFE or Dacron (rifampin soaked if possible)
- Proximal right and left subclavian arteries can be approached by median sternotomy with extension to the neck and resection of the clavicular head for exposure in a patient in extremis, a clamshell thoracotomy can also expose both vessels.
- The supraclavicular approach is through the clavicular head of sternocleidomastoid muscle and scalene fat pad with retraction of phrenic nerve and division of the anterior scalene muscle
- Place small roll under the shoulders and gently extend head away from side of injury.
Avoid injury to phrenic nerve, brachial plexus, and vertebral arteries. The proximal left subclavian artery is approached using a high (3rd intercostal space) anterolateral thoracotomy and the innominate and proximal right subclavian artery through a median sternotomy and supraclavicular incision. The innominate vein can be ligated and divided to facilitate exposure to the innominate artery. Alternatively, the mid and distal subclavian arteries on both sides can be exposed through a supraclavicular incision or combined supraclavicular/infraclavicular incisions. There is no requirement to obtain proximal vascular control within the surgical field of injury; using separate incisions through non-traumatized tissues can expedite rapid vascular control. When approaching this injury, the operator should err on the side of ample proximal exposure and if necessary, can resect the clavicular head. In an unstable patient it is recommended that initial proximal control be obtained via thoracotomy as this will allow for more rapid control than use of the supraclavicular approach. Because of the technical challenges with exposure, the utility of temporary vascular shunts in this injury pattern is limited. Most often interposition graft using 6-8 mm ePTFE or Dacron is required for subclavian artery repair, being mindful of the vertebral artery and the phrenic nerve. If endovascular capability is available, balloon occlusion of the proximal subclavian artery can be a useful adjunct, and repair with a covered stent can be considered.1,2 (see Appendix E)
- Recommendations: Repair
- Utility of temporary shunt: High (low probability of shunt thrombosis based on author’s experience)
- Method/Conduit: Interposition graft with reversed saphenous vein (favored) or ringed PTFE
- Supra- and infraclavicular incisions allows proximal control and distal exposure.
- Prep axilla, arm, and hand of upper extremity into operative field.
- Avoid brachial plexus which will be deep or lateral to axillary artery.
- Control of the proximal axillary artery is best accomplished through a supraclavicular incision, although the artery itself is exposed through an infraclavicular incision extending into the axilla. The infraclavicular exposure includes division of the clavipectoral fascia, and the blunt separation or selective division of the fibers of the pectoralis major muscle for the entire length of the wound. The axillary vein is the first structure to be encountered in the axillary sheath, inferior to the artery. The axillary artery lies superior and deep to the vein, mobilization and caudal retraction of the axillary vein will expose the first segment of the axillary artery. The first segment of the axillary artery is then visible coursing under the pectoralis minor muscle which can be retracted laterally or commonly divided. It is important when exposing the artery to have the arm and hand prepped in the operative field and extended out onto an arm board. Repair of the axillary artery most commonly involves an interposition graft using reversed saphenous vein.
- Recommendations: Repair
- Utility of temporary shunt: High (low probability of shunt thrombosis based on authors experience)
- Method/Conduit: Interposition graft with reversed saphenous vein
- Medial approach; adjacent to the median nerve in brachial sheath in bicep/triceps groove.
- Elastic artery with redundancy; flex arm slightly for interposition grafts to avoid kinking.
- Depending upon damage to collaterals, distal ligation (below profunda brachial or deep brachial artery) may be tolerated.
Brachial Artery Exposure
The brachial artery with the median nerve rests in the brachial sheath and is exposed through a medial incision in the upper arm in the groove between the bicep and triceps (see below). The median nerve is the most superficial structure encountered upon entering the brachial sheath. The ulnar nerve runs posterior to the artery which is surrounded by paired deep brachial veins. A common anatomic variant is for there to be a high bifurcation of the brachial artery in the upper third of the arm. Repair is most commonly accomplished using a reversed saphenous vein interposition graft. Although it may be possible to ligate the brachial artery below the origin of the deep (profunda) brachial artery and maintain a viable arm and hand, this proposition is based on intact collateral circulation. Unfortunately, collaterals from the shoulder and deep brachial artery are often damaged in the setting of penetrating or blast wounds and therefore maintenance of flow through the brachial artery with a temporary shunt or vascular repair is advised. Ligation or primary amputation is an acceptable damage control maneuver if there is not time for shunting or the patient is in extremis.
- Recommendations: Selective (repair some but not all)
- Utility of temporary shunt: Low (high probability of shunt thrombosis)
- Method/Conduit: Ligation, primary repair, or interposition graft with reversed saphenous vein
- The presence of an arterial doppler signal in the hand obviates the need for artery repair.
- Repair with saphenous vein in instances where the absence of an arterial signal persists.
Most often the hand has a dual arterial supply and therefore can tolerate ligation of either the radial or ulnar artery. As such, repair, or reconstruction of an injury at this level is rare. Perfusion to the hand should be assessed with Doppler before and after occlusion or ligation, and if the absence of a signal persists, reconstruction with reversed saphenous vein should be performed. Given the relatively small muscle mass of the hand and the degree of collateral circulation, ligation is most often tolerated understanding that if ischemia persists, evaluation and revascularization can be performed at a CONUS facility days or weeks later.
- Recommendations: Repair
- Utility of temporary shunt: High (low probability of shunt thrombosis based on authors experience)
- Method/Conduit: Interposition graft/ saphenous vein or 6-8mm prosthetic
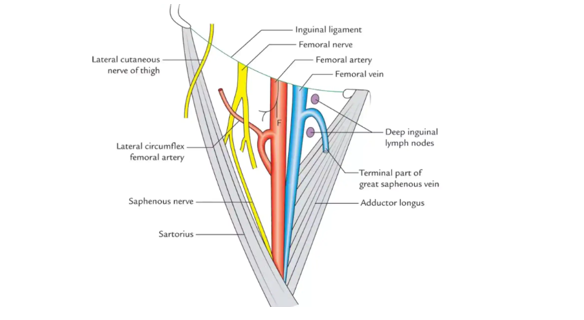
- Expose abdominal wall and artery coursing under inguinal ligament for proximal control.
- External iliac artery can be controlled through proximal groin or low abdominal incision.
- Coverage with tissue (femoral sheath), sartorius muscle or rectus flap.
Injury to the common femoral artery is often fatal as hemorrhage control in the field is difficult. Exposure is obtained through a single longitudinal incision above the artery (2-3 cm lateral to the pubic tubercle) exposing the artery at the inguinal ligament. A key point in exposing the femoral artery is ensuring there is adequate and reliable proximal and distal control prior to exploring the injury. Placing the incision proximal enough so that the abdominal wall and inguinal ligament can be identified first in a consistent and familiar location (oftentimes by drawing a line from the anterior superior iliac spine to the pubic tubercle on the skin prior to incision). Alternatively, proximal control can be obtained in the retroperitoneum (i.e. external iliac) through the proximal extension of this groin incision or by using a limited transverse incision in the lower abdomen. After a transverse-oblique skin incision, the external and internal oblique aponeuroses are divided, and the lateral fibers of the internal oblique separated. The transversus muscle and transversalis fascia are opened allowing entrance into the retroperitoneum, and the peritoneum is reflected cephalad, exposing the internal iliac vessels along the medial border of the psoas muscle. Common femoral artery injuries are commonly reconstructed using reversed saphenous vein, although e-PTFE or Dacron can be used if there is too great of a size mismatch. Placement of a prosthetic graft is acceptable if there is minimal to no contamination and there is adequate coverage. At a Role 2 facility, placing a shunt prior to transfer to a higher level of care is preferable to reconstruction with a prosthetic graft. Every attempt should be made to maintain flow into the profunda femoris artery, although the feasibility of this will depend upon the pattern of injury and the comfort level of the surgeon to perform a more complicated reconstruction. Coverage of vascular reconstruction in the groin is challenging; it may consist of local viable tissue, the sartorius muscle, or other options such as a rectus abdominis transfer flap. Coverage may be better addressed in a Role 3-4 facility. 1,3
- Recommendations: Repair if possible
- Utility of temporary shunt: Low (high probability of shunt thrombosis)
- Method/Conduit: Ligation or interposition graft with saphenous vein
- Exposure of proximal profunda is the same (distal extension) as the common femoral.
- If superficial femoral is patent, ligation of mid to distal profunda injury is acceptable.
Exposure of the proximal profunda femoris artery is obtained through a longitudinal incision used to expose the common femoral artery. Mid and distal segments are exposed through a vertical incision made parallel to the lateral border of the Sartorius muscle on the upper thigh, lateral to the proximal sartorius muscle. The sartorius is retracted medially and the rectus femoris is retracted laterally to expose the mid- and distal segments. Ligation of the circumflex profunda veins as they cross the artery is necessary. Often there are several of these crossing veins. Proximal profunda injuries should be repaired with reverse saphenous vein interposition graft. This is especially important if there is a question about the integrity of the superficial femoral or popliteal vessels. In this setting, flow through the profunda is most important to allow healing of subsequent lower extremity amputations. If patency of the superficial femoral artery can be confirmed, ligation of mid and distal profunda femoris injuries is acceptable as they lie deep in the thigh musculature and are not required for leg viability.3
- Recommendations: Repair
- Utility of temporary shunt: High (low probability of shunt thrombosis)
- Method/Conduit: Interposition graft/ reversed saphenous vein
- Medial incision with “bump” under calf, surgeon seated, OR lights over shoulder.
- Exposure of the proximal 1/3 posterior to the sartorius and distal 1/3 anterior to the Sartorius.
- Be wary of adjacent vein and geniculate branches of distal superficial femoral artery (Hunter’s canal).
Exposure is performed through a medial thigh incision and the adductors of the leg (i.e., adductor magnus). Exposure is facilitated by placing a lift or “bump” below the knee which allows the femoral artery, sartorius and adductors to be suspended, improving separation. Entry into the fascia of the lower thigh (distal superficial femoral artery) should be performed at the upper anterior margin of the sartorius which should be reflected down or posteriorly. Exposure is facilitated with the surgeon seated looking across to the dissection field with lights positioned directly over the surgeon’s shoulder if a headlight is not available.
When exposing the superficial femoral artery, it is important to recognize the femoral vein which is in proximity, if not adherent, to the artery. At the distal extent of the artery as it exits the adductor (Hunter’s) canal, there are large geniculate side branches which should be preserved or at least not injured as it causes hemorrhage. Repair of superficial femoral artery injury is best performed by reversed saphenous vein interposition graft from the uninjured leg.3
- Recommendations: Repair
- Utility of temporary shunt: High (low probability of shunt thrombosis)
- Method/Conduit: Reversed saphenous vein
- Medial incision with “bump” under calf for above knee and under thigh for below knee.
- Distal exposure by division of gastrocnemius and soleus from the tibia allowing dissection to anterior tibial origin (coursing away from dissection plane) and tibial-peroneal trunk.
- Equipment: Henley popliteal retractor with removable, varied depth side blades is valuable. Weitlaner or Cerebellar retractors, medium and small, are also helpful when Henley is not available.
- For popliteal artery intimal injuries found on initial evaluation that is not repaired at the time of injury, but instead surveilled, anti-platelet therapy is recommended (if no contraindications such as TBI) and consider repeat CTA in 48 hours for surveillance.
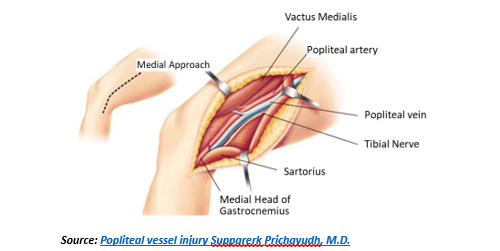
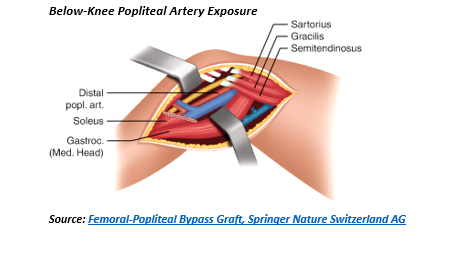
Vascular injuries in the popliteal space are exposed through a medial incision with the surgeon seated and lights over his or her shoulder. The dissection is extended from above to below the knee and is facilitated by a lift or “bump” under the calf of the leg with the knee flexed. When exposing below the knee, this bump is placed under the thigh. Natural dissection planes exist in exposing the above knee popliteal artery (i.e. popliteal space) except for the need to divide the fibers of the adductor magnus which envelop the distal superficial femoral artery (Hunter’s canal). Similarly, a natural dissection plane exists into the popliteal space from below the knee but added exposure should be accomplished by division of the gastrocnemius and soleus muscle fibers from the medial tibial condyle to allow a lengthy exposure of the below knee popliteal artery to the takeoff of the anterior tibial artery and the tibial-peroneal trunk. To completely expose the popliteal space, the medial attachments of the sartorius, semitendinosis, semimembrinosis and gracilis to the medial condyle of the tibia can be divided. When feasible, the pes anserinus should be reconstructed given its significant role in medial knee stabilization. Weitlaner, cerebellar retractors, flexible Adson-Beckman or Henly popliteal retractors with detachable side blades are necessary to expose the popliteal space. Typically, the medial head of the gastrocnemius can be retracted down using one of these devices and does not need to be divided.3
- Recommendations: Selective repair (i.e. some but not all)4
- Utility of temporary shunt: Moderate (medium probability of shunt thrombosis)
- Method/Conduit: Ligation or interposition graft with saphenous vein
- If a Doppler signal is present at the ankle, there is no need for additional tests or repair.
- Doppler exam should be repeated as patient is resuscitated and warmed.
- Repair with vein if three tibial arteries injured and an absence of a Doppler signal persists.
- As long as there is at least one of the three tibial vessels intact, injuries to one or even two of the tibial vessels can safely be managed by ligation.
- Equipment: Similar retractors to popliteal exposure. Henly popliteal retractor with removable, varied depth side blades is valuable. Weitlaner or Cerebellar retractors, medium and small, are also helpful.
- Recommendations: Selective repair (i.e. some but not all)
- Utility of temporary shunt: Moderate (medium probability of shunt thrombosis)
- Method/Conduit: Ligation, repair, or saphenous interposition graft
- Repair of proximal veins is indicated to reduce venous hypertension and congestion.
- Shunts in proximal veins will remain patent until formal repair can be performed.
- Pneumatic compression device on distal extremity to augment venous flow after repair.
Many extremity venous injuries, especially small, distal veins, can be ligated with no adverse effects because of collateral venous drainage. However, ligation of more proximal or watershed veins, or even axial veins when collaterals have been destroyed by soft tissue wounds, will result in venous hypertension and congestion. In such instances an attempt should be made to repair the vein and restore venous outflow. Temporary shunts have been shown to be effective in restoring venous outflow in the femoral veins until formal repair can be accomplished. Techniques of lateral venorrhaphy are acceptable, although an interposition graft using saphenous vein from the uninjured limb is often necessary.
The patency of vein repairs in the lower extremity is 80% at 24 months with no increased incidence of pulmonary emboli compared to ligation. Additionally, a limb salvage benefit of vein repair compared to ligation has been shown 2 years after injury.5,6 Despite these advantages, repair of extremity venous injury should only be considered in instances when the patient’s overall status is able to tolerate additional procedures; otherwise, venous ligation is preferred, despite the increase in morbidity.
Technical considerations include removing thrombus from the distal venous segments with compression (e.g., ace wrap or Esmark bandage) prior to repair. Additionally, following venous repair, placement of a pneumatic compression device distal on the extremity will augment venous flow and improve patency. Lastly, if there is no contraindication, a prophylactic dose of low-molecular weight heparin (LMWH) should be initiated or low-rate heparin drip when LMWH is contraindicated.5
ALGORITHM FOR EXTREMITY VASCULAR INJURY
References
- Clouse WD, Rasmussen TE, Peck MA, et al. Current in theater management of wartime vascular injury: a report from Operation Iraqi Freedom. J Am Coll Surg. 2007; 204(4):625-632.
- Wing W, Ji W, Wu X, Li J. Prolonged indwelling time of temporary vascular shunts is associated with increased endothelial injury in the porcine mesenteric artery. J Trauma. 2011;70:1464-70.
- Woodward EB, Clouse WD, Eliason JE, et al. Penetrating Femoropopliteal injury during modern warfare: experience of the Balad Vascular Registry. J Vasc Surg. 2008; 47:1259- 65.
- Burkhardt GE, Cox M, Clouse WD, Porras C, Gifford SM, Williams K, Walk R, Rasmussen TE. Outcomes of selective tibial artery repair following combat-related extremity injury. J Vasc Surg. 2010; 52(1):91-96.
- Quan RW, Gillespie DL, Stuart BS, Chang AS, Whittaker DR, Fox CJ. The effect of vein repair on the risk of venous thromboembolic events: a review of more than 100 traumatic military venous injuries. J Vasc Surg. 2008; 47:571-7.
- White PW, Walker PF, Bozzay JD, Patel JA, Rasmussen TE, White JM. Management and outcomes of wartime cervical carotid artery injury. J Trauma Acute Care Surg. 2020;89(2S Suppl 2):S225-S230.
APPENDIX B: TORSO VASCULAR INJURY
- Recommendations: Selective repair
- Utility of temporary shunt: None, except in extremis
- Method/Conduit: Observation and medical optimization or Dacron graft replacement
- If a stable blunt injury, MEDEVAC to Role 3 for possible repair versus MEDEVAC to Role 4.
- Permissive hypotension or B-blocker may decrease risk of rupture.
- If hemorrhage from penetrating wounds, clamshell thoracotomy and one lung ventilation to facilitate exposure of the proximal descending aorta.
Management of penetrating injury to the thoracic aorta is very rare given the pre-hospital lethality of this injury. If present, management of thoracic hemorrhage in the setting of penetrating trauma is directed by chest tube location and output (i.e. the hemithorax which is bleeding from tube thoracostomy is the one which is opened). The descending thoracic aorta is approached through the left chest and when injured is surrounded by hematoma. An initial left thoracotomy can be extended into the right chest to approach the thoracic aorta by extending across the sternum (“clam shell” thoracotomy). Aortic control proximal and distal to the hematoma must be obtained including isolation or control of any intercostal arteries in this segment. Aortic clamps are used to arrest flow in this segment and the hematoma is entered with debridement of the injured aorta using scissors. An adequate length of aorta must be debrided to allow placement of large caliber (20-26mm) Dacron graft sewn end-to-end to the proximal and distal segments.
Management of blunt injury to the thoracic aorta (partial transection or pseudoaneurysm) which has reached a temporary stable equilibrium is more common. In this setting and in the absence of hemorrhage from chest tubes, contrast CT imaging is indicated to characterize the injury. Permissive hypotension and selective use of B-blockers is indicated to decrease the risk of aortic rupture during this period. Impulse control parameters should target a goal heart rate of <80 and a systolic blood pressure of <120 mmHg. If CT confirms blunt aortic injury, options include early open repair or MEDEVAC. Blunt aortic injury may have associated thrombus or intimal injury seen on CTA and anticoagulation versus anti-platelet therapy should be considered in the context of concomitant injuries. In a patient at Role 1 or 2 with a suspected blunt aortic injury who has normal and stable vital signs and no signs of active hemorrhage from the thorax, MEDEVAC to the Role 3 should occur. At this location the decision will be made regarding options for open or endovascular repair or medical optimization and critical care transport out of theater. Recent advances in in-theater endovascular capability have made endovascular repair of such injuries possible at certain Role 3 facilities, though this is not common.1,2
- Recommendations: Repair
- Utility of temporary shunt: Low
- Method/Conduit: Interposition graft with Dacron (rifampin soaked), aortic sizers helpful in determining graft conduit diameter
- Supraceliac aortic control requires high midline incision along xyphoid, spreading and suspension of rib cage with retractors and nasogastric tube in the esophagus. Divide the entirety of the right crus overlying the aorta.
- Approach supra-mesocolic Zone I hematomas with left medial visceral rotation (Mattox maneuver).
- Approach infra-mesocolic Zone I hematomas with right medial visceral rotation (Catell-Brash maneuver).
- Keep in mind that supra-mesocolic Zone I hematomas may contain transected pancreas.
Blunt and penetrating injuries to the abdominal aorta present as a central (zone I) hematoma with blood in the abdomen at laparotomy. Zone I hematomas should be considered in two locations, supra- or infra-mesocolic, and should be entered once proximal and distal control is established and blood and access are available for transfusion. Supra-mesocolic, Zone I hematomas are best approached by left medial visceral rotation (i.e. Mattox maneuver) which exposes the supraceliac, paravisceral and infrarenal segments of aorta. Infra-mesocolic Zone I hematomas should be approached with the Catell-Brash maneuver exposing the infrarenal aorta and inferior vena cava up to and behind the liver. Proximal aortic control is obtained through the gastrohepatic ligament by retracting the esophagus to the left and dividing the crus. Alternatively, the Mattox maneuver exposes the supraceliac aorta from the lateral position, enabling proximal control as well. The iliac vessels or distal aorta can next be controlled, providing isolation before entering the hematoma. Repair techniques for the aorta and its branch vessels range from primary pledgetted closure to replacement with a Dacron interposition graft and depend upon the degree of injury.
- Recommendations: Repair
- Utility of temporary shunt: Low
- Method/Conduit: Lateral repair, patch angioplasty or interposition graft / ePTFE
- Establish resuscitation lines above the diaphragm for abdominal vena cava injuries.
- Vena cava injuries should be exposed using the Catell-Brash and Kocher maneuvers.
- Lateral repair is acceptable provided that no more than 1/3rd of the lumen is compromised.
- If occlusion of the cava results in hypotension, clamp the aorta to support central perfusion.
- Retrohepatic, retroperitoneal hematomas should not be disturbed if not actively bleeding.
- Several specific strategies applicable to repair of the injured vena cava are listed in the Large Vein Injuries section.
- Equipment: Self retaining retractor system (i.e. Omni or Bookwalter)
The approach to the vena cava in the abdomen should be performed using the Cattell-Brasch and Kocher maneuvers to expose the cava, renal veins, and the distal portion of retrohepatic segment. Mobilization of the liver is required to expose the retro-hepatic vena cava; however, retrohepatic hematomas should not be disturbed if there is no active bleeding.
Attempts should be made to identify large lumbar veins feeding into the injured segment which may bleed as much as the main channel of the vena cava if not controlled. Because repair of the vena cava is likely to require intermittent occlusion (i.e. sponge sticks or vascular clamps) or ligation in extreme cases, central venous access should be established above the diaphragm (i.e. subclavian or jugular veins) to allow effective volume resuscitation. If compressing or occluding the vena cava results in significant hypotension, the adjacent infrarenal abdominal aorta may be temporarily occluded to support central pressures while resuscitation takes place. Repair of tangential injuries to the cava can be accomplished using lateral suture repair (i.e. running venorrhaphy) provided that the lumen is not narrowed more than ½ of its native diameter. If lateral repair results in significant narrowing, there is a higher risk of thrombosis leading to pulmonary emboli and anticoagulation should be initiated postoperatively if possible. In instances where lateral repair will result in more than 50% narrowing, patch angioplasty or resection and interposition graft using ePTFE is preferable. Ligation of the infrarenal cava is acceptable as a damage control maneuver, although this carries a significant mortality risk and major morbidity in the form of decreased cardiac preload and significant lower extremity edema. If infrarenal ligation is needed, it should always be accompanied by bilateral lower leg fasciotomies to reduce the risk for compartment syndrome. Suprarenal occlusion of the IVC is generally not compatible with survival and should be considered a measure of last resort.3
PORTAL VEIN AND HEAPTIC ARTERY
- Recommendations: Repair
- Utility of temporary shunt: Low (high probability of shunt thrombosis)
- Method/Conduit: Primary repair, patch angioplasty, interposition graft / ePTFE or Dacron or saphenous vein
- Access to gastrohepatic ligament by Pringle maneuver should precede exploration of the porta hepatis.
- Ligation of hepatic artery injuries is acceptable if the portal vein is patent.
- Lateral venorrhaphy is preferred, ligation of portal vein results in massive bowel edema and systemic hypovolemia.
- Several specific strategies applicable to repair of the injured portal vein are listed in the Large Vein Injuries section (section VII).
Portal vein and hepatic artery injuries typically present as hematomas of the porta hepatis and should be explored after isolation of the gastrohepatic ligament and application of a Pringle maneuver. Next, careful dissection of the porta is performed to determine which structures have been injured. Injuries to the hepatic artery may be repaired with lateral suture placement if limited in severity; ligation of the hepatic artery is acceptable if the portal vein is uninjured. Repair of the portal vein should be attempted using the technique of lateral venorrhaphy if possible. If a large segment of the portal vein is damaged, vein patch angioplasty, or in rare instances, interposition vein graft may be performed. Ligation of the portal vein is an option of last resort and will result in hepatic ischemia and splanchnic congestion and hypervolemia for several days. Importantly, if the capabilities are available, then imaging of the biliary system should be considered for associated injuries of the common bile duct and can be performed with cholangiography through the gall bladder.
- Recommendations: Repair
- Utility of temporary shunt: Low (high probability of shunt thrombosis)
- Method/Conduit: Primary repair, patch angioplasty, interposition graft / ePTFE or Dacron or saphenous vein
- Present as supra-mesocolic Zone I hematoma.
- Repair proximal mesenteric artery and vein injuries including portal vein.
- Ligation can be performed for distal artery and vein injuries or as damage control.
Upon entering a supra-mesocolic Zone I hematoma, one may find injury to the mesenteric vessels (artery or vein). Under most circumstances, repair of the proximal superior mesenteric artery and vein, including the portal vein, is indicated using the techniques of primary pledgetted repair, vein patch angioplasty or replacement of the injured segment with interposition saphenous vein graft. The specific type of repair will depend on the location and extent of vessel injury. In cases where injury to the artery or vein is distal (i.e. beyond the middle colic artery or jejunal vein branches) or in which the patient’s physiology is severely compromised, the vessels can be ligated.
- Recommendations: Selective repair
- Utility of temporary shunt: Low
- Method/Conduit: Primary repair or patch angioplasty/Dacron or vein
- Explore Zone II hematomas from penetrating injury.
- Establish status of contralateral kidney by contrast study or manual palpation.
- Priority is “save-life,” and early nephrectomy is required with complex injuries.
- With a renal warm ischemic time > 30-60 minutes, complex repairs are not indicated.
- Injury to the renal pedicle (blunt or penetrating) is closely associated with injury to the parenchyma; isolated arterial injury is rare. Essential considerations in the management of renal artery injury in wartime are the warm ischemic time of 30-60 minutes and complexity of renal artery repair, as times longer than this has dismal renal preservation rates. Both of these limits what the surgeon can accomplish in the context of renal artery injury other than ligation and nephrectomy.
- If arterial injury manifests as occlusion with renal ischemia, it will be too late to restore flow and function to the kidney by the time the diagnosis is made in the operating room or CT scanner. If the artery is patent and bleeding, an associated Zone II or lateral hematoma requiring exploration will be present and attempts to stop hemorrhage and repair are indicated. These maneuvers may include pledgetted primary repair of the renal artery, patch angioplasty or very rarely interposition graft replacement (aorto-renal bypass). Again, considering the warm ischemic time of the kidney, complex operations to maintain or reestablish perfusion in the renal artery are not recommended and should be abandoned in favor of nephrectomy in most cases.
- The method by which to approach an expanding or penetrating Zone II hematoma is controversial and case specific. Isolation of the renal pedicle before exploring the hematoma is doctrine in many institutions and has the advantage of aortic isolation and definitive proximal control. However, from a practical standpoint, mobilization of the damaged kidney from a lateral to medial direction without hilar control may be faster depending upon the appearance of the injury. The lateral to medial approach is like the medial visceral rotation performed for Zone I injuries.
- Recommendations: Repair
- Utility of temporary shunt: High (low probability of shunt thrombosis)
- Method/Conduit: Interposition graft/ ePTFE or Dacron or saphenous
- Explore Zone III hematoma from penetrating wound after establishing aortic control above the hematoma.
- Distal control is obtained at the inguinal ligament (i.e. external iliac arteries).
- Wylie hypogastric (internal iliac) clamps facilitate low-profile control of iliac arteries.
Iliac artery injuries generally present as a Zone III or pelvic hematoma with or without extremity ischemia (check femoral pulses). Exploration of the hematoma should be performed after proximal control is obtained at the infrarenal aorta, the contralateral iliac artery if possible, and ipsilateral distal external iliac artery. The distal external iliac artery should be located as it exits the pelvis at the inguinal ligament at a point where it is free from the hematoma. The internal iliac artery may not be initially controlled or visualized before exploring the hematoma, which often requires opening to expose the internal iliac. The inability to initially control all bleeding from the hematoma necessitates preparation including multiple suction devices, Fogarty occlusion balloons (if available) direct tamponade strategies or devices and alerting anesthesia regarding the need for continued resuscitation during exploration. After proximal and distal control of the common and external iliac arteries is obtained, the hematoma is entered which facilitates exposure and clamping of the internal iliac artery and the injured vessel(s). Common and external artery injuries can be controlled and managed with a temporary vascular shunt if needed or repaired with interposition grafting using saphenous vein or prosthetic conduit (6-8mm ePTFE or Dacron). In an unstable patient or a patient where there is contamination of the field, shunt placement with definitive repair or reconstruction done at a later point is a good option.
If the primary injury is to the internal iliac artery (hypogastric), it may be ligated with 3.0 or 4.0 Prolene on an SH needle. Bleeding from associated iliac veins may be severe and difficult to expose. The iliac artery may be divided, if necessary, to facilitate exposure of the iliac vein, followed by repair of the artery. At certain Role 3 facilities with endovascular capabilities, selective embolization of bleeding hypogastric artery or branches is an option, particularly in blunt trauma (e.g., pelvic fracture.) The principles which apply to the management of iliac vein injury are discussed in the Management of Large Vein Injuries Section.
- Feliciano DV. Management of traumatic retroperitoneal hematoma. Ann Surg. 1990; 211:109-123.
- Propper BW, Alley JB, Gifford SM, Burkhardt GE, Rasmussen TE. Endovascular treatment of a blunt aortic injury in Iraq: extension of innovative endovascular capabilities to the modern battlefield. Ann Vasc Surg. 2009;23(5):687.e19-687.
- Sullivan PS, Dente CJ, Patel S, et al. Outcome of ligation of the inferior vena cava in the modern era. Am J Surg 2009;199:500-6.
APPENDIX C: CERVICAL VASCULAR INJURY
- Recommendations: Repair.
- Utility of temporary shunt: High (low probability of shunt thrombosis and restores flow to the brain quickly)
- Method/Conduit: Vein patch or vein interposition graft.
- Zone I cervical injuries are best approached with median sternotomy for ample proximal exposure.
- Early control of common carotid with umbilical tape/Rummel or DeBakey clamp.
- 3 Fr Fogarty with 3-way stopcock is useful to occlude internal carotid back bleeding.
- Shunt and augment mean arterial pressure during carotid repair to perfuse brain.
- Equipment: Weitlaner or Cerebellar self-retaining retractors, Argyle or Sundt shunts, and vascular clamps such as angled DeBakey clamp, profunda clamp, Kitzmiller clamp. Javid clamp.
Step 1: Place shunt into internal carotid (or distal carotid) and secure with Javid clamp or Rummel; allow back bleeding.
Step 2: Place the shunt through the lumen of the vein graft.
Step 3: Insert shunt into proximal carotid and secure with Javid clamp or Rummel.
Step 4: Restore of forward flow through the shunt then perform the distal vein graft anastomosis using 6-0.
Step 5: Start the proximal anastomosis to the common carotid with 6-0 Prolene.
Step 6: When the anastomosis is nearly completed, the shunt is removed through the remaining anastomotic opening.
Step 7: Remove the proximal aspect of shunt from the common carotid and observing back bleeding from the shunt in the internal carotid.
*Care should be taken to ensure the vein graft is reversed to negate the function of the venous valves. Vein valves may inhibit back bleeding through the vein graft.
Most carotid injuries result from penetrating wounds and result in hematoma. Indications for operation are bleeding or injury with interrupted flow (i.e. occlusion). When feasible, contrast CT should be performed for neck wounds. CT aids in the triage for urgent operation, improves operative planning and images the brain as a baseline. Although a selective approach to exploration of Zone II neck wounds is acceptable, if a carotid injury is identified, the neck should be explored, and an attempt made to repair. The exceptions are blunt injury resulting in carotid occlusion greater than 12 hours or a Zone III injury not accessible by standard techniques.
Exposure of the carotid artery is through a generous incision coursing anterior to the sternocleidomastoid and facilitated by a roll under the shoulders, extension of the neck and turning of the head away from the injury. The platysma is divided, and the sternocleidomastoid muscle reflected posterolaterally. The internal jugular vein is carefully dissected and mobilized laterally, exposing the carotid artery. The carotid is exposed proximal to the hematoma and controlled with an umbilical tape into a Rummel device (i.e. red rubber catheter). In the absence of uncontrolled bleeding, there is no need to tighten the Rummel; but having it in place gives one this option and allows for securing the proximal end of a temporary shunt.
The dissection proceeds distal into the zone of injury. If bleeding is encountered the Rummel may be cinched or a clamp (angled DeBakey) slid proximal to the umbilical tape using it to pull the carotid up into the clamp, thereby avoiding injury to the vagus nerve. Back bleeding from the internal carotid artery is a favorable sign and can be controlled with a small clamp or a (3 Fr) Fogarty inserted into the internal and inflated using a 1cc syringe and 3-way stop-cock to maintain inflation. The external carotid artery is controlled with vessel loops or ligated. If the internal and common carotid arteries are controlled above and below the injury, a temporary shunt can be placed to maintain perfusion while the injury is identified, and options considered. First, the shunt should be placed into the internal carotid artery and secured with a vessel loop or small Javid shunt clamp allowing back bleeding through the shunt. To secure the proximal shunt, an angled DeBakey is placed proximal to the umbilical tape and Rummel device. Then in sequence, the shunt is placed in the common carotid through the Rummel which is partially tightened around the shunt. As it is advanced deeper (more proximal) into the common carotid, the DeBakey clamp is slowly opened allowing the shunt to pass while the Rummel is tightened down fully securing the shunt in place. Alternatively, the common carotid artery can be controlled with fingers as the shunt is inserted proximal and the Rummel synched down. If available, Javid shunt clamps can be used to occlude the artery around the shunt instead of the Rummel device. A similar technique can be utilized with vessel loops to secure the shunt.
Repair of carotid artery injuries most commonly requires placement of an interposition saphenous vein graft, although primary repair or vein patch angioplasty can be performed for less severe injuries. To perform the interposition graft over the shunt, the proximal end is removed using the DeBakey clamp to again occlude the common carotid proximal to the Rummel. The vein graft is next placed over the shunt (i.e. shunt in the vein graft lumen). The proximal shunt is reinserted into the common carotid and secured with the Rummel using previously described sequence. After flow is restored in the shunt, the distal vein graft anastomosis is performed using 6-0 Prolene to the edge of the normal internal carotid. Next the proximal anastomosis to the common is started also with 6-0 Prolene. When the anastomosis is nearly completed, the shunt is removed through the remaining anastomotic opening, first removing the proximal from the common carotid and observing back bleeding from the shunt in the internal carotid. Finally, the shunt is removed from the internal and the vein graft flushed generously with heparinized saline and the anastomosis completed. Alternatively, the reconstruction can be performed without a shunt, however, this exposes the ipsilateral hemisphere to prolonged ischemia. Regardless of whether a shunt is used, the mean arterial pressure should be kept above 90mmHg during the repair to optimize cerebral perfusion. In more experienced surgical hands, measuring of an internal carotid stump pressure with SBP >50mmHg indicates intact Circle of Willis and collateralized hemispheric flow, allow easier repair of the carotid injury with interposition.
Alternatively, the reconstruction can be performed without a shunt, however, this exposes the ipsilateral hemisphere to prolonged ischemia. Regardless of whether a shunt is used, the mean arterial pressure should be kept above 90mmHg during the repair to optimize cerebral perfusion.
If no other life-threatening injuries are present, a small amount (50u/kg) of systemic heparin is recommended along with generous flushing of the repair with heparinized saline to prevent platelet aggregation and clot formation. Ligation of the internal carotid artery is an acceptable damage control maneuver to stop hemorrhage but has an acute stroke rate of 30-50% according to historic data, with more recent data showing a stroke rate of 100% after ligation due to penetrating trauma.1
- Recommendations: Ligate
- Utility of temporary shunt: None
- Method/Conduit: N/A
- Bleeding vertebral artery injuries are ligated with no role for reconstruction in theater.
- Vertebral artery occlusions are managed with anticoagulation if it is not contraindicated.
- Endovascular embolization is an option if injury is not accessible by standard exposure.
Repair of vertebral artery injuries in wartime is extremely rare and most commonly bleeding from this vessel is ligated as a matter of necessity during neck exploration. Alternatively, vertebral artery injury (occlusion or extravasation) can be discovered on a contrast CT scan. There should be a high index of suspicion for this injury with cervical spine fractures. In instances of acute vertebral artery occlusion, anticoagulation is recommended to reduce the risk of posterior circulation stroke although the clinical evidence to support this is limited. If associated injuries preclude use of systemic heparin, then antiplatelet therapy should be initiated.
- Recommendations: Selective repair
- Utility of temporary shunt: None
- Method/Conduit: Lateral venorrhaphy, vein patch or saphenous vein
- Significant jugular vein injuries can be ligated without adverse effects.
- Repair of jugular injuries should be considered in the setting of TBI with elevated ICP.
- Repair instead of ligation of jugular vein injuries may be indicated in instances of associated closed head injury to reduce intracranial pressures, although little data exists to support this practice. Minor jugular vein injuries can be repaired by lateral suture repair (venorrhaphy) while patch angioplasty or interposition vein graft are options for more extensive injuries. Operative exposure of the jugular vein is the same as that described for the carotid artery.
Sullivan PS, Dente CJ, Patel S, et al. Outcome of ligation of the inferior vena cava in the modern era. Am J Surg 2009;199:500-6.
APPENDIX D: MISCELLANEOUS TOPICS
- Intervention should be avoided in those less than 10 years old given propensity for spasm.
- Ligation is more well tolerated in infants and toddlers given ability to recruit collaterals.
- Perform interrupted suture lines (6-0 Prolene) to allow expansion with growth of child.
Although rare, deployed surgeons can expect to see young patients with vascular injury. Intervention of any type, including angiography, should be avoided in those less than 5 years even if an extremity appears ischemic (i.e. without Doppler signal). The small size of arteries in children and their propensity for vasospasm makes it more likely that an intervention will do harm or confuse the clinical scenario rather than improve the situation. Because of the ability of children to tolerate relative limb ischemia and to develop collateral circulation, ligation of bleeding vessels alone is recommended with warming of the extremity and resuscitation. In rare cases, in children older than 8, reconstruction of larger proximal arteries can be accomplished using reversed saphenous vein. In such instances, the anastomosis should be performed using interrupted suture allowing expansion as the child grows.1
ENDOVASCULAR CAPABILITY & INFERIOR VENA CAVA FILTERS
(See Trauma-Specific Endovascular Inventory Tables) 2
- Techniques should be used in a small subset of injuries and directed by a trauma surgeon.
- Indications for vena cava filter include inability to initiate chemoprophylaxis within 48 hours of injury and the occurrence of pulmonary embolus while on chemoprophylaxis.
The emergence of catheter based endovascular technology to manage injury in the civilian setting has been expanded to the wartime setting. Although advantageous in a small set of combat injuries, endovascular capability in austere settings is in its early stages and its application should be directed by appropriately trained surgeons or interventional radiologists. Injury patterns and procedures which lend themselves to endovascular techniques include central injuries of the thoracic aorta and brachiocephalic vessels (subclavian and carotid) and select patterns of solid organ and pelvic injury amenable to coil embolization. Placement of vena cava filters to reduce the risk of pulmonary thromboembolic events is indicated in patients who cannot receive chemoprophylaxis or therapy with heparin. A trauma specific endovascular inventory for in-theater capability is listed in Trauma-Specific Endovascular Inventory Tables on the next page.
Indications for placement of an inferior vena cava filter include an inability to initiate chemoprophylaxis with low molecular weight heparin or unfractionated heparin due to contraindications, proximal deep vein thrombosis and contraindications for full anticoagulation, failed trial of anticoagulation for DVT or pulmonary embolism (progression or bleeding). Examples of contraindications to chemoprophylaxis include significant traumatic brain, solid organ, or pelvic injuries with bleeding (refer to JTS Prevention of Venous Thromboembolism CPG). The Günther-Tulip (Cook Medical, Inc.) filter is currently recommended because of its established record of success and its ability to be removed in certain circumstances. (See tables on the next page.)
APPENDIX E: TRAUMA - SPECIFIC ENDOVASCULAR INVENTORY TABLES
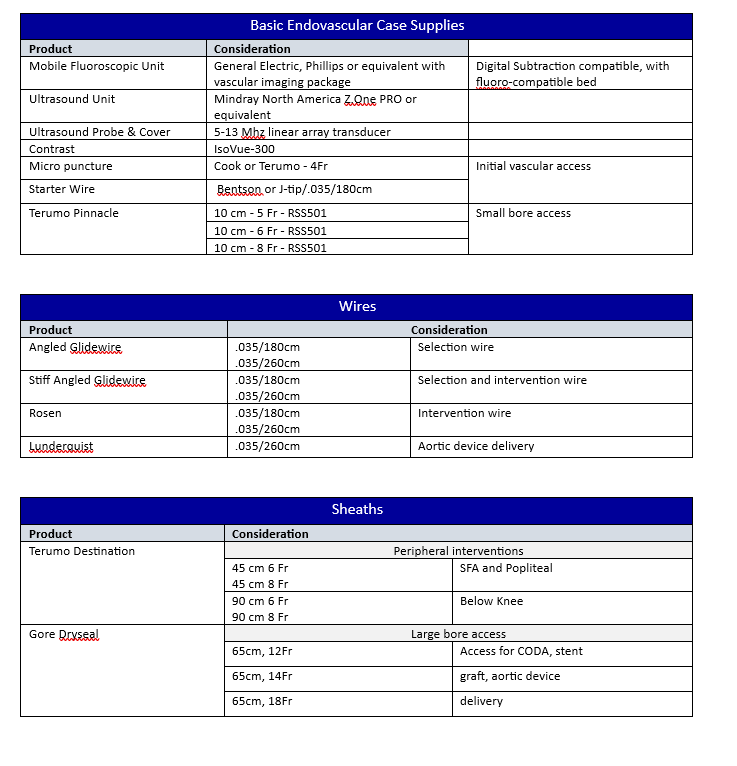
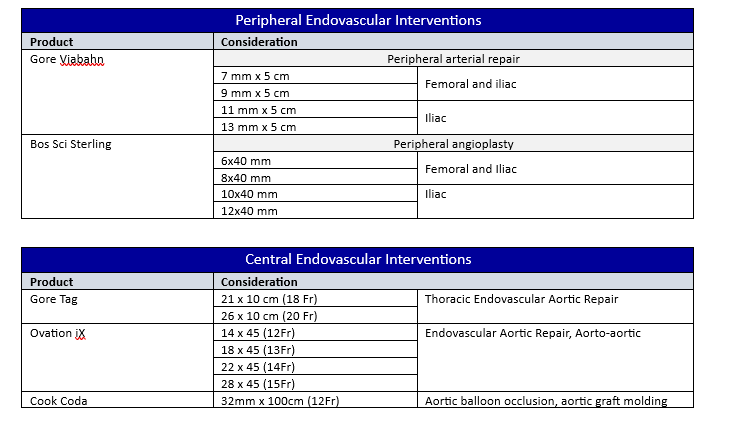
- Clouse WD, Rasmussen TE, Perlstein J, Sutherland MJ, Jazerevic S. Upper extremity vascular injury: a current in theater wartime report from Operation Iraqi Freedom. Ann Vasc Surg. 2006; 20(4):429-434.
- Cox MW, Whittaker DR, Martinez C, Fox CJ, Feuerstein IM, Gillespie DL. Traumatic pseudoaneurysm of the head and neck: early endovascular intervention. J Vasc Surg 2007; 46(6): 1227-1233.
APPENDIX F: CLASS VIII MEDICAL MATERIEL


APPENDIX G: TELEMEDICINE/TELECONSULTATION
APPENDIX H: INFORMATION REGARDING OFF-LABEL USES IN CPGS
The purpose of this Appendix is to ensure an understanding of DoD policy and practice regarding inclusion in CPGs of “off-label” uses of U.S. Food and Drug Administration (FDA)–approved products. This applies to off-label uses with patients who are armed forces members.
Unapproved (i.e. “off-label”) uses of FDA-approved products are extremely common in American medicine and are usually not subject to any special regulations. However, under Federal law, in some circumstances, unapproved uses of approved drugs are subject to FDA regulations governing “investigational new drugs.” These circumstances include such uses as part of clinical trials, and in the military context, command required, unapproved uses. Some command requested unapproved uses may also be subject to special regulations.
Additional Information Regarding Off-Label Uses in CPGs
The inclusion in CPGs of off-label uses is not a clinical trial, nor is it a command request or requirement. Further, it does not imply that the Military Health System requires that use by DoD health care practitioners or considers it to be the “standard of care.” Rather, the inclusion in CPGs of off-label uses is to inform the clinical judgment of the responsible health care practitioner by providing information regarding potential risks and benefits of treatment alternatives. The decision is for the clinical judgment of the responsible health care practitioner within the practitioner-patient relationship.
Consistent with this purpose, CPG discussions of off-label uses specifically state that they are uses not approved by the FDA. Further, such discussions are balanced in the presentation of appropriate clinical study data, including any such data that suggest caution in the use of the product and specifically including any FDA-issued warnings.
With respect to such off-label uses, DoD procedure is to maintain a regular system of quality assurance monitoring of outcomes and known potential adverse events. For this reason, the importance of accurate clinical records is underscored.
Good clinical practice includes the provision of appropriate information to patients. Each CPG discussing an unusual off-label use will address the issue of information to patients. When practicable, consideration will be given to including in an appendix an appropriate information sheet for distribution to patients, whether before or after use of the product. Information to patients should address in plain language: a) that the use is not approved by the FDA; b) the reasons why a DoD health care practitioner would decide to use the product for this purpose; and c) the potential risks associated with such use.





