Whole Blood Transfusion
Joint Trauma System
Whole Blood Transfusion
Definitions
Whole blood (WB) collected in the anticoagulants CPD or CPDA-1 is an FDA-approved product when it is appropriately collected, stored and tested for transfusion transmitted disease (TTD) by a licensed blood donor center. It can be stored for 21 days at 1-6°C in CPD or 35 days at 1-6°C in CPDA-1 and is designated stored whole blood (SWB) in this CPG. SWB retains in vitro hemostatic parameters to an acceptable level during approved storage duration;1 however, after the first 2 weeks of storage, the hemostatic function of WB may vary and supplementation with fresher whole blood units or blood components, especially platelets, may be necessary.
Fresh whole blood (FWB) refers to WB collected on an emergency basis from a “walking blood bank” (WBB). FWB can either be stored at room temperature and used within 24 hours of collection (and then destroyed if not used) or it can be refrigerated within 8 hours of collection, after which point it becomes WBB-SWB. FWB is considered to have full hemostatic function. FWB is collected from pre-screened donors when possible, but does not undergo TTD testing prior to transfusion; this fact makes it not approvable by the FDA. Because FWB presents a higher risk of disease transmission, it is reserved for situations in which tested blood products are unavailable or ineffective (further discussion below).
The most important safety consideration in transfusing WB is that donor red blood cells (RBCs) be compatible with the recipient to avoid acute hemolytic transfusion reactions (a.k.a., major mismatch). WB from group O donors contains RBCs that are compatible with all recipients, but the plasma in group O WB may contain anti-A and anti-B antibodies that could cause hemolysis in a non-group O recipient (a.k.a., minor mismatch). There are two approaches to mitigating this risk: 1) transfuse only group-specific WB (i.e., A to A, B to B, AB to AB and O to O), or 2) anti-A and anti-B antibody titers can be measured in group O WB and only units containing a low titer of antibody (e.g., <1:256 saline dilution, immediate spin method) are designated “low titer O WB” (LTOWB) and these are used as “universal WB.” LTOWB has been used extensively to resuscitate combat casualties and was a standard of care in WWII, and the conflicts in Korea and Vietnam.2 Note that LTOWB may be either SWB or may be collected from pre-screened O donors in a WBB protocol and thus be considered FWB (e.g., the Ranger O Low titer or ROLO protocol).3 In practice, the only SWB supplied by the Armed Services Blood Program (ASBP) to OCONUS locations will be LTOWB due to the relatively higher risk of donor-recipient blood group mismatch and resulting hemolysis during group-specific WB transfusion, compared to the much lower risk of hemolysis with LTOWB.2 Collecting LTOWB from WBB pre-screened donors is also preferred to group-specific transfusion. In short, most WB transfused during future contingency operations will be LTOWB, and most of this is likely to be SWB. Use of LTOWB is recognized under AABB Standard 5.15.1 (31st Edition, AABB Standards, in effect beginning 01 April 2018).4
It should be noted that anti-A and anti-B titers may vary in group O donors. Ideally, WBB donors should be re-titered every 90 days in conjunction with TTD testing. However; since availability of titer testing in the deployed setting is very limited, every effort should be made to ensure that donors are titered at least annually if not prior to each deployment. ASBP collects WB from male and never-pregnant female donors, or from female donors testing negative for anti-HLA antibodies (this mitigates risk of transfusion-associated acute lung injury, TRALI). WB is primarily collected from Rh positive donors and there is a limited supply of Rh negative blood products in the deployed environment. Every effort should be made to provide Rh negative whole blood or red cells to females of child-bearing potential (age<50 years) who are Rh negative or of unknown blood type. However; should transfusions of Rh positive blood products occur in these patients, these must be thoroughly documented in the patient’s medical record due to the risk of allo-immunization to Rh and potential for hemolytic disease of the fetus/newborn (HDFN) in future pregnancies.
All WB products (SWB, FWB, and LTOWB) are indicated for the resuscitation of massive blood loss. WB, and in particular LTOWB, is the preferred resuscitation product for the pre-hospital treatment of patients in hemorrhagic shock.5,6 This CPG will distinguish between stored whole blood (SWB) and fresh whole blood (FWB), and discuss uses and limitations of both products.
BACKGROUND
The first documented animal-to-animal (dog) blood transfusion was performed at Oxford in 1665 by Richard Lower, followed by the first animal-to-human blood transfusion in 1667 by Jean Denis. The first human-to-human blood transfusion was performed by James Blundell in 1818. In the year 1900, the ABO blood grouping system was classified by Landsteiner and, based on this, the first pre-transfusion cross-match was done by Ottenberg in 1907. The system of Rh typing was invented by Landsteiner and Wiener in the year 1940.7 In military settings, whole blood has been used extensively to resuscitate casualties in military conflicts since 1917, during World War I.8 Whole blood is the starting point for blood donation and continues to be used extensively worldwide where component production is not available.
Blood safety and sustainability are global issues. Component development supports the sustainability of blood services where demand can outstrip supply. Component use also permits optimal storage conditions for each element of the blood, minimizes hemolytic reactions and supports precision treatment. Examples include the use of red blood cells (RBCs) for anemia, fresh frozen plasma (FFP) to replace lost or consumed clotting factors, platelets (PLTs) for platelet abnormalities and thrombocytopenia, and cryoprecipitate (Cryo) for hypofibrinogenemia. Whole blood contains all of these elements in a smaller volume of anticoagulant and thus provides a more concentrated product for treating bleeding patients who need all elements of blood replaced. The widespread use of component therapy is driven by blood product availability. For the reasons outlined above, blood banks have preferred to stock components over WB.
The clinical data comparing WB to components have recently been reviewed.6 Currently available clinical data indicate that use of WB to treat hemorrhage results in outcomes that are at least as favorable as those that can be expected with component therapy that includes RBCs, plasma and platelets.
Severely injured combat casualties requiring transfusion have a significant mortality rate (16%) and have the greatest potential to benefit from early and appropriate transfusion strategies. A large retrospective cohort study of casualties requiring transfusions during Operations Iraqi Freedom (OIF) and Enduring Freedom (OEF) suggests a significant survival benefit for transfused casualties when RBCs, fresh frozen plasma, and platelets are transfused at a 1:1:1 ratio.9 A recent randomized trial in civilian trauma patients demonstrated that a 1:1:1 transfusion ratio resulted in improved early hemostasis, though no statistically significant improvement in survival.10 Two retrospective analyses in combat casualties comparing FWB to component therapy (which included platelets) have also been published. One study showed a potential survival benefit to the use of FWB during resuscitation of severe combat injuries, and the other showed FWB to be equivalent to component therapy.11,12 These studies underscore the importance of providing all elements of whole blood (RBCs, plasma and platelets) to severely bleeding patients and suggest that use of either WB or components in a 1:1:1 ratio for resuscitation of bleeding patients is acceptable; product choices can be guided by practical considerations.
Advantages of Whole Blood Over Components
SWB and FWB provide FFP:RBC:PLTs in a physiologic ratio and return to the bleeding patient what has been lost. It should be noted that the 1:1:1 ratio of blood components (platelets: plasma:RBC) recommended for damage control resuscitation does not faithfully reconstitute WB. The 1:1:1 ratio yields a dilute blood mixture with a hematocrit of 29%,13 a platelet count of approximately 90,000/µL, and coagulation factors diluted to approximately 62% of WB concentrations due to the presence of anticoagulants and red cell additive solution. In addition, WB delivers all needed elements of blood in only one product, which only requires refrigeration for storage. In contrast, component therapy requires multiple products and storage modalities (refrigeration, freezing and generally room temperature storage with agitation for platelets – though platelets can also be refrigerated), greatly increasing workload and complexity for clinical teams.
SWB collected in licensed blood centers offers the same level of TTD safety as component therapy collected in licensed centers. It should be noted that due to the extremely short shelf life of standard room temperature stored platelets (5 days), all platelet products transfused in the deployed setting are collected in theater and do not undergo TTD testing prior to transfusion. Therefore, SWB collected in licensed centers and fully tested presents a lower TTD risk than component therapy using in-theater collected platelets or FWB.
For U.S. casualties presenting in hemorrhagic shock, a transfusion strategy that included FWB with RBCs and plasma was associated with an improved survival compared to the use of stored components only (FFP, RBCs, and PLTs).11 Compared to SWB or component therapy, FWB is more readily available in austere conditions and requires only the presence of donors and simple collection equipment, though safe collection and transfusion of FWB requires appropriate pre-deployment training 14,15 and careful donor evaluation. FWB has no loss of the labile clotting factors or platelet activity that is often associated with storage, has close to physiological hematocrit and has no red blood cell “storage lesion”. Storage lesion describes the degradation of the RBC involving loss of membrane plasticity,11,12 diphosphoglycerate, adenosine triphosphate, nitric oxide, and other factors leading to potentially reduced delivery of oxygen to tissues and contribution to a variety of pathophysiologic processes.16 It should be noted that recent randomized trials assessing the effects of red blood cell storage age have not confirmed a clinically detectable deleterious effect of the red cell storage lesion in the populations evaluated. The effect of red cell storage age, whether in component therapy or SWB has not been rigorously evaluated in certain vulnerable populations, such as trauma patients.17
Overall, both SWB and FWB offer at least comparable performance and safety compared with components, as well as compelling logistical advantages that are particularly important in pre-hospital resuscitation and indeed, in most deployment settings.
Considerations in Choosing SWB or FWB
There are risks associated with the use of FWB, including but not limited to increased risk of transfusion-transmitted infections (e.g., HIV, hepatitis B/C, syphilis), and an increased risk of clerical errors leading to major mismatch when ABO-identical WB is provided, due to the potentially chaotic conditions during which FWB is requested.
Additionally, field conditions are inherently unsanitary and are presumed to increase the risk of bacterial contamination of the blood. Recent history with approximately 10,000 FWB transfusions to U.S. personnel during OIF/OEF have resulted in one Hepatitis C (HCV), one Human T-Lymphocyte Virus (HTLV) seroconversion, and one fatal case of transfusion-associated graft-versus host disease that was potentially due to a FWB transfusion.4 FWB is not FDA-approved and is not intended or indicated for routine use. It is NOT appropriate, as a matter of convenience, to use FWB as an alternative to more stringently controlled blood products for patients who do not have severe, immediately life-threatening injuries. FWB is to be used only when other blood products cannot be delivered at an acceptable rate to sustain the resuscitation of an actively bleeding patient, when specific stored products are not available (e.g., SWB, RBCs, FFP, PLTs, Cryo), or when stored components are not adequately resuscitating a patient with an immediately life-threatening injury. FWB should not routinely be collected from pre-screened donors as a way to maintain a routine inventory of WBB-SWB products. In other words, the use of WBB for collection of FWB is for emergency use only. It should be noted that studies of FWB donors have not documented significant decrements in military-relevant task performance following donation. Thus, concerns that FWB collections will adversely affect mission outcomes have not been substantiated and should not preclude WBB activation when conditions for FWB use are met.18
In patients receiving LTOWB (SWB or FWB), every effort should be made to obtain a pre-transfusion blood sample in order to establish the original blood group. If blood samples are obtained after transfusion with LTOWB, it may be impossible to definitively establish a patient’s blood group with the equipment available in the deployed setting. As a result, patients of unknown blood group receiving LTOWB will continue to receive LTOWB or group O RBC units for their acute transfusion requirements for up to a month following admission. This can deplete inventories of LTOWB and group O RBCs.
Whole Blood Recommendations
- SWB, which will in practice be LTOWB, is the preferred product for pre-hospital resuscitation.
- In a facility capable of providing surgical care (Role 2 or higher), SWB (in practice, LTOWB) or component therapy (including RBCs, plasma and platelets) can be used for damage control resuscitation. SWB simplifies transfusion and may facilitate more rapid resuscitation of casualties, and may enhance a facility’s capacity to manage MASCAL challenges.
- The use of FWB should be reserved for casualties with clinically significant shock or coagulopathy (e.g. bleeding with associated metabolic acidosis, thrombocytopenia or INR>1.5) and when SWB or optimal component therapy (e.g. apheresis platelets and FFP) are unavailable, or when stored component therapy is not adequately resuscitating a patient with immediately life-threatening injuries.
Guidelines for Walking Blood Bank Program for FWB
The decision to use FWB is a medical decision that must be made by a physician who has full knowledge of both the clinical situation and the availability of compatible blood products. A Walking Blood Bank (WBB) Program should be established based on a risk assessment and the potential for casualties. The calculation of risk should include a medical intelligence assessment which includes infection prevalence and the need for preventative force protection measures. In practice, all forward-deployed MTFs should establish a WBB. Coordination with the Area Joint Blood Program Officer (AJBPO) is required to establish a WBB Program. (Appendix B: Blood Donor Pre-screening Standard Operating Procedure [SOP]). FWB should be collected for transfusion as outlined in Appendix C: Emergency Whole Blood Drive SOP. In general, the use of FWB should be limited to casualties who are anticipated to require a transfusion when the physician determines that SWB or optimal component therapy is unavailable or in limited supply, or in patients that are not responding to SWB or component therapy. The decision to initiate a FWB drive should be made in consultation with the appropriate MTF medical authority (e.g., Deputy Commander for Clinical Services (DCCS), Trauma Director, Trauma Surgeon) and Laboratory/Blood Bank OIC. At Role 2 facilities, the lead surgeons and/or facility OIC should be consulted on the decision to initiate the drive.
Pre-screened donors registered into the WBB Program are preferably composed of active duty, active reserve, active National Guard, and other DoD beneficiaries. The preferred donors for FWB are fully pre-screened, low titer O donors. Next, consider fully pre-screened donors of other blood groups for group-specific transfusions (e.g., A to A). Donors who have not been pre-screened for TTDs should be considered only when no other donors are available. Note that in chaotic circumstances such as tactical care under fire or mass casualty (MASCAL) scenarios, or if blood grouping equipment is not available in adequate quantities, use of group O FWB of unknown anti-A and anti-B titer may be safer than attempting to match blood groups between donors and recipients, since the risk of hemolysis from major mismatch is greater than the risk of transfusing a very high titer group O unit (very high titers units being relatively uncommon) to a non-group O recipient. Indeed, this strategy was successfully employed by a Forward Surgical Team in Afghanistan.19
Donors should be screened to international mandated and national standards. Coalition Forces will not be utilized routinely as donors, due to national variances in screening for blood borne diseases and differences in disease prevalence. Blood may be collected from pre-screened coalition partner forces if the screening program has been reviewed by the JBPO and deemed acceptable by the COCOM Surgeon and the ASBP Director. Planned coalition activity should address the interoperability of donor panels. Non-Coalition Force foreign nationals should be used as a last resort.
The decision to use FWB that has not been completely screened for infectious agents is a medical decision that must be made after thorough consideration of risks and benefits. Decision-making should be adequately documented in the casualty record.
The blood type on identification tags is occasionally incorrect (last correlated data equated to about 4% inaccurate) 20-22 and must not be relied upon routinely to determine blood type for either donors or recipients. Identification tags for ABO/Rh verification should be utilized as a last resort only.
Use of non-standard blood donation material and equipment may lead to coagulation during the collection process potentially causing an adverse transfusion reaction; therefore, only authorized equipment will be utilized (Appendix C enclosure: WBB Supply List [with NSNs]).
Prior to issuing FWB for transfusion, the ABO and Rh type should be verified and approved rapid infection disease tests (e.g., HIV, HCV, and HBV) should be performed as outlined in Appendix C: Emergency Whole Blood Drive SOP to the greatest extent possible.
Theater Medical Data Stores (TMDS), Blood Portal, shall be utilized to record FWB donations and infectious disease testing results.
Frequency of FWB donation must be tracked. In general, WB units should not be collected from donors more frequently than every 8 weeks (56 days). This interval between donations is important to allow the donor to recover RBC mass and iron stores and should not be shortened except under the most extreme circumstances. Donors who give blood frequently may develop iron deficiency even in the absence of anemia. Iron deficiency can cause fatigue, difficulty concentrating, pica, restless leg syndrome (RLS), and eventually anemia if untreated. Iron deficiency can be diagnosed by measuring serum ferritin levels (deficiency defined as ferritin <30 mcg/L in males and <20 mcg/L in females). In deployed settings, it may be impossible to measure ferritin levels but donors at particular risk of iron deficiency include: young donors (to early 20’s), premenopausal females, frequent donors (males ≥ 3x/year, females ≥ 2x/year), and donors near hemoglobin cutoff for donation (males 13.0 g/dL, females 12.5 g/dL). Consideration should be given to screening ferritin prior to deployment in high risk donors, particularly low titer O donors who may be called upon to donate more frequently. Consideration should be given to empiric iron supplementation in high risk donors or donors with symptoms of iron deficiency (available as ferrous sulfate 325mg (65mg elemental iron), ferrous gluconate 325 mg (38mg elemental iron), or multivitamins with iron (18-19 mg elemental iron); one tablet per day for 60-120 days may be adequate to replete iron stores).23,24 Patients with documented iron deficiency (low ferritin levels as above) should be offered iron supplementation and monitored for response.
WBB Planning
Since the need for FWB cannot be predicted, a robust contingency operational plan should be developed by the MTF staff to include the Laboratory/Blood Bank and surgical and anesthesia providers in coordination with the Area Joint Blood Program Officer. The plan should be reviewed and rehearsed regularly. Equipment and consumables should be inspected with due attention paid to storage conditions and expiry dates.
The key elements for planning and readiness to administer FWB are knowledge and rehearsal of two SOPs: Blood Donor Pre-Screening (Appendix B) and Emergency Whole Blood Drive (Appendix C).
- A contingency plan should be developed for collecting, storing, and transfusing FWB in MASCAL situations or when it may be deemed that the current blood inventory will be exhausted prior to re-supply (e.g., when multiple type-O trauma casualties are exhausting the type-O RBC inventory).
- The physical donation site should be organized in such a way as to maintain the integrity of the screening and donation process, and to minimize the possibility of clerical errors. This is especially important in emergency situations involving more than one casualty.
- Every effort should be made to adhere to the same screening, drawing, labeling, and issuing standards required for U.S. FDA-approved blood products.
- Pre-screened donors in the WBB Program determined to be suitable should be utilized, to the greatest extent possible, before using personnel who: (1) have been pre-screened or donated in the past but do not have current (within 90 days) screening and infectious disease testing; (2) have no pre-screen or donation history. All donors must be rescreened at the time of donation.
- Use LTOWB donors if available. Otherwise, upon determining the ABO/Rh status of the casualty, activate the WBB Program, re-calling pre-screened donors with the same ABO/Rh using the TMDS>Manage Donor>View Donor List, if available, or other record keeping systems. All donors should have their ABO/Rh verified (i.e. Eldon card or laboratory testing) at the time of donation. Titers for LTOWB donors should be obtained pre-deployment, which should be no more than 12 months prior to donation. The ABO and RhD group should be the same as that on the dog tag and records. Before any FWB is transfused, rapid infectious disease testing (i.e. HIV, HBV, HCV) of donor specimens shall be performed, to the greatest extent possible.
- Retrospective samples must be sent to a licensed laboratory for FDA-approved testing, regardless of whether the rapid infectious disease testing is performed pre- or post-transfusion, as these tests are not licensed for donor testing.
- Upon the notification of confirmed positive infectious disease results, a medical provider or preventive medicine personnel will be notified to ensure that the donor is notified and counseled. Donors and unit commanders must understand the importance of donor tracing.
- If a patient receives a confirmed positive infectious disease unit, the AJBPO will notify the Armed Services Blood Program immediately to initiate patient notification and an evaluation of both the donor and patient.
- In accordance with HA Policy 10-002, Policy on the Use of Non-U.S. Food and Drug Administration, recipients of FWB shall receive follow-up advice and infectious disease testing as soon as possible, and at 3-, 6-, and 12-months post-transfusion.
- Procedure. See Appendix B enclosure: ASBP 572–EWB (Emergency Whole Blood).
- Only one unit of FWB should be collected per donor. In situations where there are a limited number of donors and a dire need for blood, no more than two units may be taken from a donor. Performance decrements may occur after two-unit collections and volume resuscitation of the donor may be necessary. Collection of more than one unit per donor should only be considered under extreme circumstances and these should be thoroughly documented.
WB Pediatric Considerations
WB has been administered to pediatric patients in recent conflicts.25 WB has not been rigorously studied in pediatric trauma resuscitation, but has been shown to reduce blood loss and transfusion requirements in pediatric cardiac surgery.11
There are no established clinical criteria for administration of WB in bleeding pediatric patients. Physiologic variables should be interpreted by age (e.g. hypotension = systolic blood pressure < 70 + 2*age in years).
For patients <40kg, WB should be delivered in “unit doses” of 10-15 ml/kg. WB is more readily volume-titrated than component therapy. There are no known contraindications to using WB in pediatric casualties.
A massive transfusion in children is defined as 40 ml/kg (total blood volume is approx. 70-80ml/kg).25
Performance Improvement (PI) Monitoring
POPULATION OF INTEREST
- All patients who receive blood product transfusion within 3 hours of injury.
- All patients who meet criteria for blood transfusion (severe traumatic injury (ISS ≥16 and ≥ 2 body regions injured with AIS severity ≥ 2 AND SBP < 100 OR HR > 100 OR hematocrit < 32% OR pH <7.25 within 3 hours of injury)
INTENT (EXPECTED OUTCOMES)
- SWB, particularly LTOWB, is used when available for pre-hospital resuscitation.
- SWB or component therapy is routinely used for damage control resuscitation and FWB is reserved for casualties who meet one of these two criteria:
- Patients with clinically significant shock or coagulopathy (e.g., bleeding with associated metabolic acidosis, thrombocytopenia or INR >1.5) when SWB or optimal component therapy (e.g., PLTs and FFP) are unavailable
- SWB or component therapy is not adequately resuscitating a patient with immediately life-threatening injuries.
PERFORMANCE/ADHERENCE MEASURES
- SWB was used in prehospital resuscitation.
- SWB or component therapy was routinely used for damage control resuscitation.
- FWB was used for casualties who fall into one of these two criteria:
- Patients with clinically significant shock or coagulopathy (e.g., bleeding with associated metabolic acidosis, thrombocytopenia or INR >1.5) when SWB or optimal component therapy (e.g., PLTs and FFP) was unavailable
- SWB or component therapy was not adequately resuscitating the patient with immediately life-threatening injuries.
DATA SOURCE
- Patient Record
- DoD Trauma Registry
- Blood transfusion databases
SYSTEM REPORTING & FREQUENCY
The above constitutes the minimum criteria for PI monitoring of this CPG. System reporting will be performed annually; additional PI monitoring and system reporting may be performed as needed.
The system review and data analysis will be performed by the Joint Trauma System.
References
- Pidcoke HF, McFaul SJ, Ramasubramanian AK, et al. Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion. 2013 Jan;53 Suppl 1:137S-149S.
- Strandenes G, Berséus O, Cap AP, et al. Low titer group O whole blood in emergency situations. Shock. 2014 May;41 Suppl 1:70-5.
- Fisher AD, Miles EA, Cap AP, Strandenes G, Kane SF. Tactical Damage Control Resuscitation. Mil Med. 2015 Aug;180(8):869-75. doi: 10.7205/MILMED-D-14-00721.
- Yazer MH, Cap AP, Spinella PC. Raising the Standards on Whole Blood. J Trauma Acute Care Surg. 2017 Dec 28
- Butler FK, Holcomb JB, Schreiber MA, et al. Fluid Resuscitation for Hemorrhagic Shock in Tactical Combat Casualty Care: TCCC Guidelines Change 14-01--2 June 2014. J Spec Oper Med. 2014 Fall;14(3):13-38.
- Spinella PC, Cap AP. Whole blood: back to the future. Curr Opin Hematol. 2016 Nov;23(6):536-542.
- Arya RC, Wander GS, and Gupta, P. Blood component therapy: Which, when and how much. J Anaesthesiol Clin Pharmacol. 2011 Apr-Jun; 27(2): 278–284.
- Stansbury LG, Hess JR. The 100th anniversary of the first blood bank. Transfusion. 2017 Nov;57(11):2562-2563.
- Pidcoke HF, Aden JK, Mora AG, et al. Ten-year analysis of transfusion in Operation Iraqi Freedom and Operation Enduring Freedom: increased plasma and platelet use correlates with improved survival. J Trauma Acute Care Surg. 2012 Dec;73(6 Suppl 5):S445-52.
- Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. PROPPR Study Group. JAMA. 2015 Feb 3;313(5):471-82.
- Spinella PC, Perkins JG, Grathwohl JG, et al. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66:S69-S76.
- Perkins JG, Cap AP, Spinella PC, et al. Comparison of platelet transfusion as fresh whole blood versus apheresis platelets for massively transfused combat trauma patients (CME). Transfusion. 2011 Feb;51(2):242-52.
- Hess JR. Resuscitation of trauma-induced coagulopathy. Hematology Am Soc Hematol Educ Program. 2013;2013:664-7.
- Strandenes G, De Pasquale M, Cap AP, et al. Emergency whole-blood use in the field: a simplified protocol for collection and transfusion. Shock. 2014 May;41 Suppl 1:76-83.
- Doughty H, Thompson P, Cap AP, et al. A proposed field emergency donor panel questionnaire and triage tool. Transfusion. 2016 Apr;56 Suppl 2:S119-27.
- Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014 Jul;107(1):1-9.
- Belpulsi D, Spitalnik SL, Hod EA. The controversy over the age of blood: what do the clinical trials really teach us? Blood Transfus. 2017 Mar;15(2):112-115.
- Strandenes G, Skogrand H, Spinella PC, et al. Donor performance of combat readiness skills of special forces soldiers are maintained immediately after whole blood donation: a study to support the development of a prehospital fresh whole blood transfusion program. Transfusion. 2013 Mar;53(3):526-30.
- Nessen SC, Eastridge BJ, Cronk D, et al. Fresh whole blood use by forward surgical teams in Afghanistan is associated with improved survival compared to component therapy without platelets. Transfusion. 2013 Jan;53 Suppl 1:107S-113S.
- Rentas FJ1, Clark PA. Blood type discrepancies on military identification cards and tags: a readiness concern in the U.S. Army.Mil Med. 1999 Nov;164(11):785-7.
- Frohman EM. Blood typing errors on U.S. army identification cards and tags. Mil Med. 1989 May;154(5):273-4.
- Gaydos JC, Polk AJ, Cowan DN, et al. Blood typing errors on U.S. Army identification (ID) cards and tags. Mil Med. 1990 Apr;155(4):A19.
- Mast AE, Bialkowski W, Bryant BJ, Wright DJ, Birch R, Kiss JE, D’Andrea P, Cable RG, Spencer BR. A randomized, blinded, placebo-controlled trial of education and iron supplementation for mitigation of iron deficiency in regular blood donors. Transfusion 2016;56:1588-1597. PMID: 26813849
- Bialkowski W, Kiss JE, Wright DJ, Cable RG, Birch R, D’Andrea P, Bryant BJ, Spencer BR, Mast AE. Estimates of total body iron indicate 19 mg and 38 mg oral iron are equivalent for the mitigation of iron deficiency in a longitudinal study. American Journal of Hematology 2017; 92:851-857. PMID: 28494509).
- Repine TB, Perkins JG, Kauvar DS, Blackborne L. The use of fresh whole blood in massive transfusion. J Trauma. 2006;60:S59-S69.
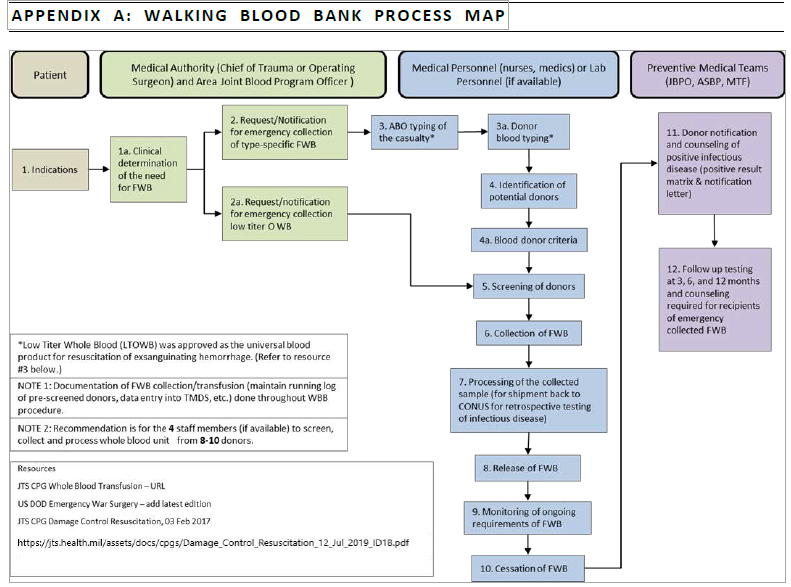
Appendix A: Walking Blood Bank Process Map
Appendix D: Additional Information Regarding Off-Label Uses in CPGs
Purpose
The purpose of this Appendix is to ensure an understanding of DoD policy and practice regarding inclusion in CPGs of “off-label” uses of U.S. Food and Drug Administration (FDA)–approved products. This applies to off-label uses with patients who are armed forces members.
Background
Unapproved (i.e. “off-label”) uses of FDA-approved products are extremely common in American medicine and are usually not subject to any special regulations. However, under Federal law, in some circumstances, unapproved uses of approved drugs are subject to FDA regulations governing “investigational new drugs.” These circumstances include such uses as part of clinical trials, and in the military context, command required, unapproved uses. Some command requested unapproved uses may also be subject to special regulations.
Additional Information Regarding Off-Label Uses in CPGs
The inclusion in CPGs of off-label uses is not a clinical trial, nor is it a command request or requirement. Further, it does not imply that the Military Health System requires that use by DoD health care practitioners or considers it to be the “standard of care.” Rather, the inclusion in CPGs of off-label uses is to inform the clinical judgment of the responsible health care practitioner by providing information regarding potential risks and benefits of treatment alternatives. The decision is for the clinical judgment of the responsible health care practitioner within the practitioner-patient relationship.
Additional Procedures
Balanced Discussion
Consistent with this purpose, CPG discussions of off-label uses specifically state that they are uses not approved by the FDA. Further, such discussions are balanced in the presentation of appropriate clinical study data, including any such data that suggest caution in the use of the product and specifically including any FDA-issued warnings.
Quality Assurance Monitoring
With respect to such off-label uses, DoD procedure is to maintain a regular system of quality assurance monitoring of outcomes and known potential adverse events. For this reason, the importance of accurate clinical records is underscored.
Information to Patients
Good clinical practice includes the provision of appropriate information to patients. Each CPG discussing an unusual off-label use will address the issue of information to patients. When practicable, consideration will be given to including in an appendix an appropriate information sheet for distribution to patients, whether before or after use of the product. Information to patients should address in plain language: a) that the use is not approved by the FDA; b) the reasons why a DoD health care practitioner would decide to use the product for this purpose; and c) the potential risks associated with such use.







