Eye Trauma: Initial Care
Joint Trauma System
SUMMARY OF CHANGES
- Antibiotics for open globe and intraocular foreign body (IOFB)
- Aeromedical evacuation considerations
- Lateral canthotomy, inferior cantholysis technique with instructional pictures
BACKGROUND/EXECUTIVE SUMMARY
- Eye injuries are common in warfare.1-3 During Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) 10-15% of combat-related trauma injuries involved the eye. 4
- Significant numbers of potentially serious eye conditions can arise in non-battle environments and during non-battle activities.1
- A large percentage of serious and/or blinding eye injuries can be prevented by wearing approved combat eye protection4 from the Authorized Protective Eyewear List (APEL) available at https://www.peosoldier.army.mil/equipment/eyewear/.
- The eye is extremely intolerant of injury.
- Eye trauma requires prompt evaluation and treatment by an eye surgeon.
- The foundation for successful treatment and preservation of vision is most often laid in the initial phases by forward medical providers at all echelons of care.
- Ophthalmic resources are deployed increasingly sparingly in very select locations.
- Initiate teleophthalmology consultation with eye surgeon as soon as possible. (See Teleophthalmology.)
- Evacuate all vision-threatening injuries so that they receive treatment by an eye surgeon within 24 hours, if possible.
- Factors such as combat operations, aircraft availability, weather, proximity to port, transport safety and security can affect the timing of transfer and definitive treatment.
- When evacuation to an eye surgeon is delayed or caring for ocular injuries in the Prolonged Field Care Environment, see the Joint Trauma System Ocular Injuries and Vision-Threatening Conditions in Prolonged Field Care Clinical Practice Guideline, 01 Dec 2017.
GOALS OF EYE TRAUMA CARE
PRIORITIES
Maintain high index of suspicion based upon the mechanism of injury.
Eye Exam
- When possible, assess and document, at a minimum, visual acuity.
- Examine for critical findings. (See below.)
Management
- Recognize and immediately treat the two traumatic eye emergencies listed below:
- For chemical injury, irrigate immediately and copiously. (See Chemical Injury.)
- For orbital compartment syndrome, perform immediate lateral canthotomy and inferior cantholysis. (See Orbital Compartment Syndrome.)
- SHIELD AND SHIP vision-threatening eye injuries:
- Protect injured eyes (known or potential injuries) with a rigid eye shield immediately (i.e. “Eyepro” or “Fox shield”).
- Maintain patient comfort, treat pain and nausea.
- Prevent further damage.
- Do NOT put pressure on eye with suspected open globe injury.
- Initiate teleophthalmology consultation with eye surgeon as soon as possible. (See Teleophthalmology.)
- Urgent: Call Role 3 or Role 4 with an eye surgeon (ophthalmologist).
- Non-urgent: Contact Role 3 or Role 4 with an eye surgeon (ophthalmologist). Can use use DHA Global Teleconsultation Portal web-based system if no ophthalmologist in theatre.
- Evacuate all vision-threatening injuries so that they are able to receive treatment by an eye surgeon within 24 hours when possible.
Prevent Eye Injuries
-
- Encourage wearing only approved eye protection in accordance with unit guidelines and requirements. Examples: Military Combat Eye Protection (MCEP), Ballistic Protective Eyewear (BPE), “eyepro,” or “eye armor.” (See Protective Eyewear.)
- Discourage contact lens use.
EYE HISTORY
“Keep an eye out” for eye trauma.
- Maintain high index of suspicion based upon mechanism of injury.
- Blast injury - most common cause eye injury in OIF and OEF.5,6
- Direct facial and eye trauma.
- Cranial or brain injury.5,7
- Metal on metal mechanisms (metallic fragments can penetrate without obvious signs on exam).
- Compressive blunt force trauma (can cause ruptured globe).
- Multisystem trauma. It is easy to overlook ocular trauma.
- Unconscious patient who cannot report vision change.
- Thermal burns, especially to the face.
- If patient can follow commands, ask about eye symptoms such as vision loss, decreased vision, double vision, or eye pain.
- Ask if casualty was wearing MCEP, BPE, “Eyepro,” or “eye armor” at time of injury. Review past eye history, including use of glasses or contact lens.
- Review tetanus status.
INITIAL EYE EXAM
VISION
- Assess and document visual acuity for each eye if possible. (See Visual Acuity Testing.) 8
- Presenting vision may be the best predictor of final visual outcome.9
Intraocular Pressure (IOP)
- Do NOT put pressure on eye with suspected open globe injury.
- If orbital compartment syndrome is suspected, palpate eyelids to see if one eye has increased firmness and resistance compared with the opposite eye (“rock hard” eyelids).
- Do not attempt to check IOP at Role 1 or 2 unless experienced with this technique.
Pupils
Test for relative afferent pupillary defect (RAPD) using swinging flashlight test. (See RAPD Testing.)
Extraocular motility
If patient can follow commands, ask to follow your finger without moving the head. Record any restrictions to movement.
Examination
Examine for critical findings. Perform focused eye exam “outside to inside.”
- External Exam – face, bony orbit, eyelids.
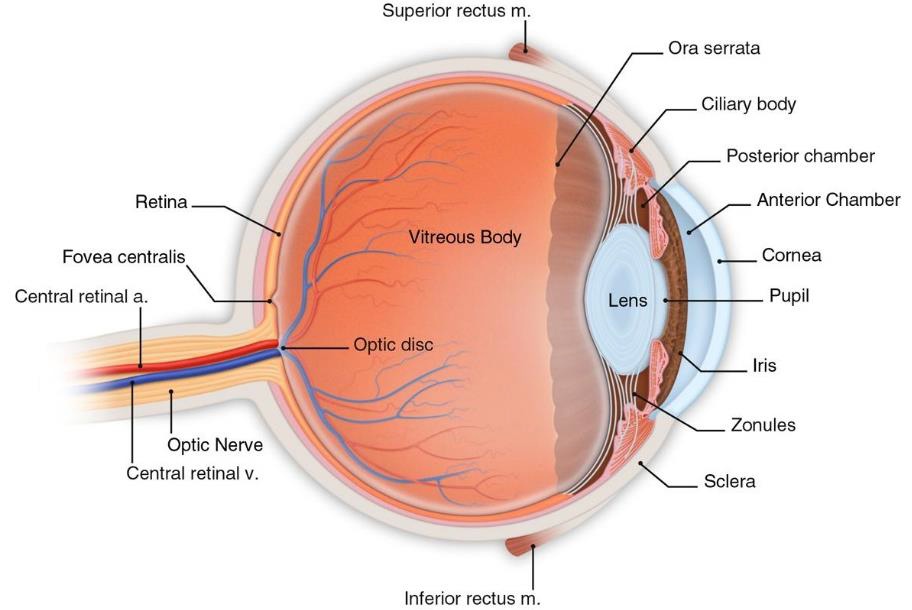
- Eye – conjunctiva, cornea, anterior chamber, iris, lens. (See Eye Anatomy.)
- Unless experienced, do not attempt fundus/posterior segment examination at Role 1 or 2.
- Do NOT attempt ultrasound of the eye. It places pressure on the eye.
SHIELD AND SHIP
SHIELD AND SHIP vision-threatening eye injuries 10-11
- Protect injured eyes (known or potential) with a rigid eye shield immediately - Eyepro or Fox shield. (See How to Place Eye Shield.)
- Base protection choice upon findings of eye history and exam.
- Treat eyelid injuries like an open globe.
- Maintain patient comfort during transport.
- Prevent Valsalva which can increase the risk of extrusion of intraocular contents.
- Provide pain control and sedation as needed.
- Treat nausea and vomiting aggressively (Ondansetron 4-8 mg IV).
- Avoid strenuous movements.
- Bed rest with head elevated 30 degrees if possible.
- Prevent further damage. Do no harm. (See Do No Harm Section below.) Do NOT put pressure on eye with suspected open globe injury.
- Initiate teleophthalmology consultation with eye surgeon as soon as possible. (See Teleophthalmology.) Provide focal history, exam, and photographs when able.
- Evacuate all vision-threatening injuries so that they are able to receive treatment by an eye surgeon within 24 hours if possible.
- Teleophthalmology consultation with an ophthalmologist is recommended before fixed wing transport (see Aeromedical Transport Considerations).
- When in doubt: SHIELD AND SHIP.
DO NO HARM (DO NOT’S)
- DO NOT let a suspected eye injury leave your level of care without rigid eye protection.
- DO NOT patch. It puts pressure on eye. (DO shield but DO NOT patch.)
- DO NOT wrap. It puts pressure on eye.
- DO NOT place anything under an eye shield, including gauze.
- DO NOT put pressure on eye with suspected open globe injury; it may increase the risk of extrusion of intraocular contents.
- DO NOT check intraocular pressure at a Role 1 or 2. (Eye surgeon will check at Role 3 or 4.)
- DO NOT attempt ultrasound of the eye. It places pressure on the eye.
- DO NOT remove impaled or resistant foreign bodies.
- DO NOT attempt to repair eye.
- DO NOT enucleate or debride tissue, even if eye is severely traumatized.
TELEOPHTHALMOLOGY
- Management of eye injuries is complex. Recommendation is to engage an eye surgeon via teleophthalmology consultation as soon as possible.
- Teleophthalmology improves and extends ophthalmic care to remote deployed locations.
- Urgent: Use phone or video teleconference service to call a Role 3 or Role 4 with an eye surgeon (ophthalmologist).
- Non-urgent: Non-urgent: Contact Role 3 or Role 4 with an eye surgeon (ophthalmologist). Can use DHA Global Teleconsultation Portal web-based system if no ophthalmologist in theatre.
- Information to provide in consult:
- Focal history: Mechanism of injury, history of eyepro use, use of glasses or contact lenses
- Focal exam: Visual acuity, key exam findings
- Picture(s)
PREVENTION
- Encourage wearing only approved eye protection with MCEP, BPE, “eyepro,” or “eye armor” (protective eyewear per Unit guidelines).
- Discourage contact lens use.
Figures 1 & 2. Eye armor will help prevent many, but not all, eye injuries. If any kind of injury involves any portion of the face that would otherwise be covered by protective spectacles or goggles, maintain high level of suspicion – regardless of obvious severity.
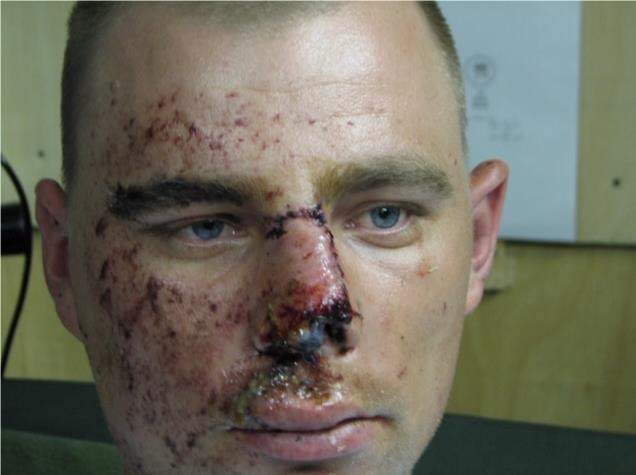
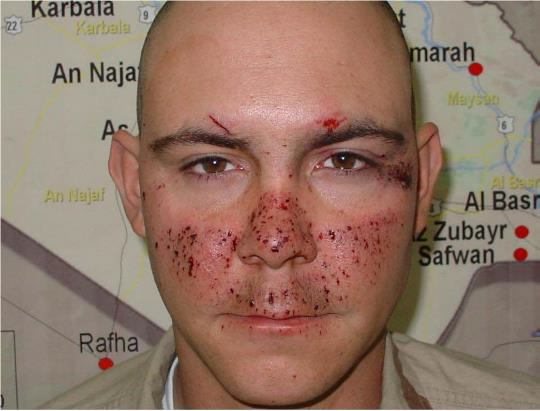
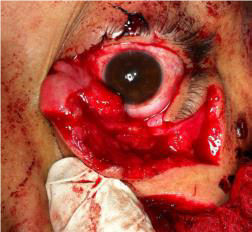
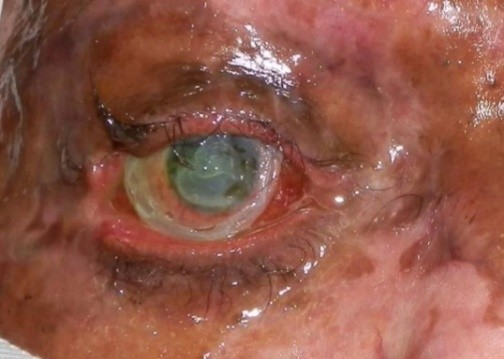
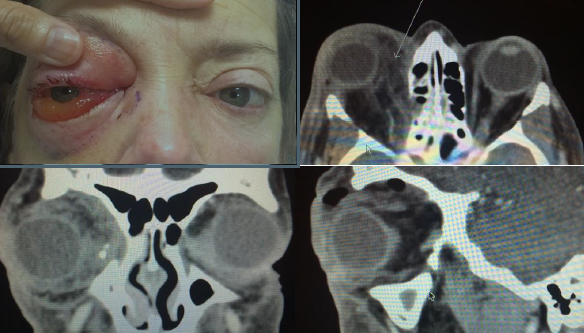
OPEN GLOBE INJURY
MECHANISM
- Laceration(s) from penetrating or perforating trauma. 13
- Ruptured globe from blunt trauma.
EXAM FINDINGS
- Collapsed or severely distorted eye.
- Open wound, full-thickness corneal or scleral laceration.
- Shallow anterior chamber.
- Peaked or irregular pupil.
- Prolapse of intraocular contents outside the eye. Dark tissue is iris or uveal tissue.
- Subconjunctival hemorrhage (SCH), especially if 360 degrees.
MANAGEMENT 14-19
- Assess and document vision when possible.
- Early teleophthalmology consultation with eye surgeon (See Teleophthalmology).
- SHIELD AND SHIP
- Rigid eye shield immediately.
- Evacuation for urgent surgical repair within 24 hours when possible. 18,20-25
- Nothing to eat or drink.
- Bed rest with head elevated 30 degrees if possible.
- Avoid maneuvers that increase intraocular pressure.
- Start systemic antibiotics. 14,26-34
- Levofloxacin 750 mg IV/PO q24hrs PLUS Vancomycin 15-20mg/kg IV q8-12hrs OR
- Moxifloxacin 400 mg IV/PO q24hrs
- Preferred regimen for prophylaxis is levofloxacin plus vancomycin or moxifloxacin alone based on limited data, expert opinion, and spectrum of antibiotic activity; this provides coverage for B. cereus, gram negative organisms including Pseudomonas aeruginosa, and drug resistant gram positive organism such as MRSA. Note that vancomycin penetration into vitreous is limited in a healthy eye; weight based dosing may improve levels. Levofloxacin and moxifloxacin have excellent penetration. Levofloxacin has superior gram negative coverage and moxifloxacin has greater gram positive coverage. Either drug, when used alone, do not have reliable MRSA coverage.
- Treat nausea and vomiting aggressively (Ondansetron 4-8 mg IV).
- Administer tetanus prophylaxis per Infection Prevention in Combat-Related Injuries CPG.
- Provide sedation and analgesia as needed (maintain patient comfort).
- DO NOT put pressure on eye. It may increase the risk of extrusion of intraocular contents.
- DO NOT attempt ultrasound of the eye. It places pressure on the eye.
- DO NOT attempt to repair.
- DO NOT attempt to enucleate eye.
- DO NOT attempt to debride tissue, even if the eye is severely traumatized.
Figures: Open globe.
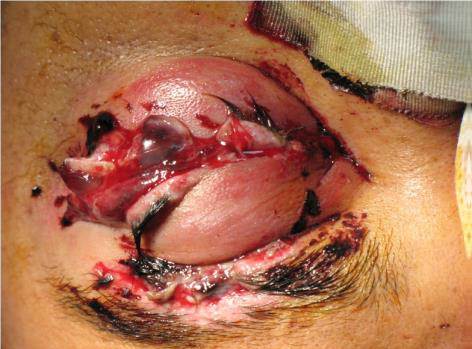
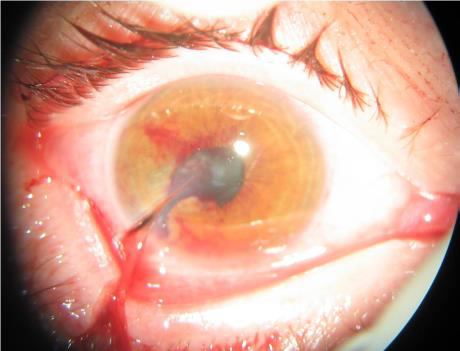
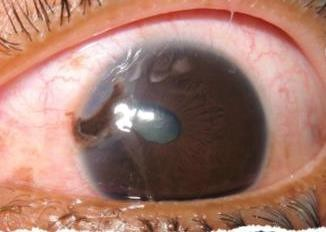
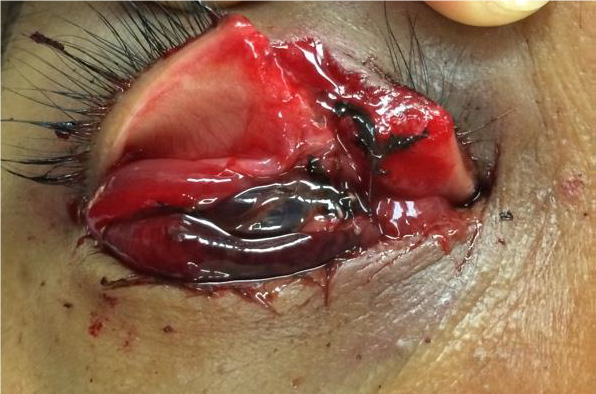
INTRAOCULAR FOREIGN BODY (IOFB) INJURY
HISTORY
High index of suspicion if blast injury, shrapnel, or metal-on-metal injury.
EXAM
- Penetrating or perforating site(s) sclera or cornea.
- Hole in iris.
- Findings may be subtle; maintain high index of suspicion.
MANAGEMENT 37,38
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology.)
- SHIELD AND SHIP
- Apply rigid eye shield immediately.
- Evacuate for urgent surgical repair within 24 hours if possible. 38-44
- Nothing to eat or drink.
- Bed rest with head elevated 30 degrees if possible.
- Avoid maneuvers that increase intraocular pressure.
- Start systemic antibiotics. 38,40
- Levofloxacin 750 mg IV/PO q24hrs PLUS Vancomycin 15-20mg/kg IV q8-12hrs OR
- Moxifloxacin 400 mg IV/PO q24hrs
- Preferred regimen for prophylaxis is levofloxacin plus vancomycin or moxifloxacin alone based on limited data, expert opinion, and spectrum of antibiotic activity; this provides coverage for B. cereus, gram negative organisms including Pseudomonas aeruginosa, and drug resistant gram positive organism such as MRSA. Note that vancomycin penetration into vitreous is limited in a healthy eye; weight based dosing may improve levels. Levofloxacin and moxifloxacin have excellent penetration. Levofloxacin has superior gram negative coverage and moxifloxacin has greater gram positive coverage. Either drug, when used alone, do not have reliable MRSA coverage.
- Treat nausea and vomiting aggressively (Ondansetron 4-8 mg IV).
- Administer tetanus prophylaxis per Infection Prevention in Combat-Related Injuries CPG.
- Provide sedation and analgesia as needed.
- DO NOT attempt to remove IOFB.
- DO NOT put pressure on eye, it may increase the risk of extrusion of intraocular content.
Figures: Intraocular foreign body. Findings may be subtle.
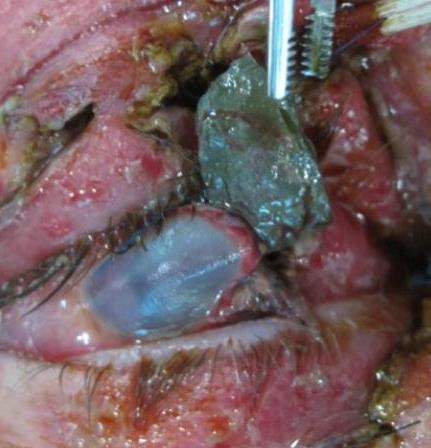

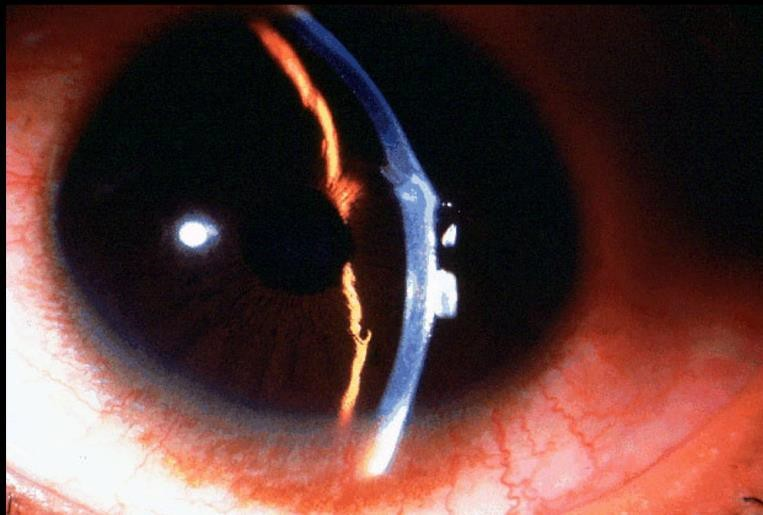
CHEMICAL INJURY
EXAM
- Epithelial defect(s) of cornea or conjunctiva
- Redness and swelling of conjunctiva
- Blanching of conjunctiva
- Corneal opacification
MANAGEMENT
- Begin irrigation immediately.
- Irrigate with normal saline or lactated ringers if available.
- Acceptable to use water or any neutral irrigation solution (best solution choice is normal saline or lactated Ringer's).
- Use Morgan Lens (first choice if available) or nasal cannula hooked to IV tubing for continuous irrigation. (See Continuous Irrigation Using Nasal Cannula.)
- Use a minimum of 2 liters irrigation if unable to check PH. Some chemical injuries require up to 10 liters.
- Apply topical anesthesia with tetracaine, proparacaine, or lidocaine as needed to maintain patient comfort during irrigation.
- DO NOT try to neutralize acid with base or base with acid.
- Remove visible acidic or basic foreign bodies with a cotton tip applicator (CTA).
- Remember to inspect the conjunctival fornices for retained foreign bodies. Irrigate or sweep fornices with a CTA.
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology.)
- SHIELD AND SHIP.
Figures: Chemical injury.
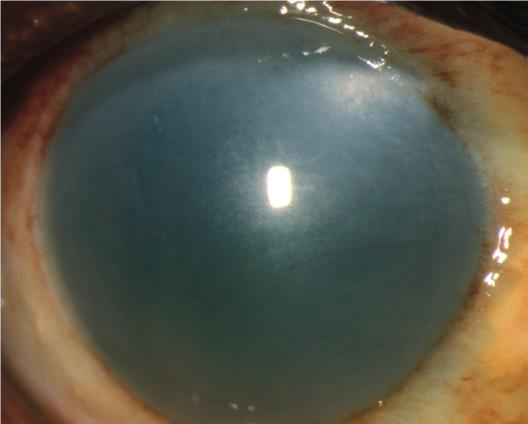
ORBITAL COMPARTMENT SYNDROME
HISTORY
Most common cause is retrobulbar hemorrhage/orbital hemorrhage.
EXAM
- Decrease or loss of visual acuity.
- “Rock hard” eyelids, or eyelids tight against the globe on palpation. Palpate eyelids to see if one eye has increased firmness and resistance compared with the opposite eye.
- Proptosis: bulging of affected eye compared to the other.
- Relative afferent pupillary defect (RAPD).
MANAGEMENT
- Emergent lateral canthotomy and inferior cantholysis. (See Lateral Canthotomy.)
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology).
- SHIELD AND SHIP.
HYPHEMA
EXAM
- Blood or clot in anterior chamber.
- “8-ball” or “black ball” hyphema is blood filling the anterior chamber.
MANAGEMENT
- Rule out open globe.46
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology.)
- SHIELD AND SHIP.
- Bed rest with head elevated 30 degrees if possible.
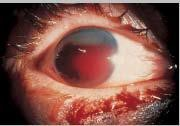
ORBITAL FRACTURE
EXAM
- Step-off orbital rim.
- Restricted eye movements.
- Enophthalmos (affected eye is further back in orbit compared with other eye).
- Hypoglobus (affected eye is lower compared with other eye).
- Subcutaneous or conjunctival emphysema.
- Numbness below eye.
MANAGEMENT
- Urgent repair required when clinical evidence of extraocular muscle entrapment with nonresolving bradycardia, heart block, nausea, vomiting, or syncope (oculocardiac reflex).
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology).
- SHIELD AND SHIP.
- Consider systemic antibiotics if sinusitis or dirty wound.
- Avoid Valsalva and maintain sinus precautions (no nose blowing).
- Maintain high suspicion for open globe.
Figures: Orbital fracture.
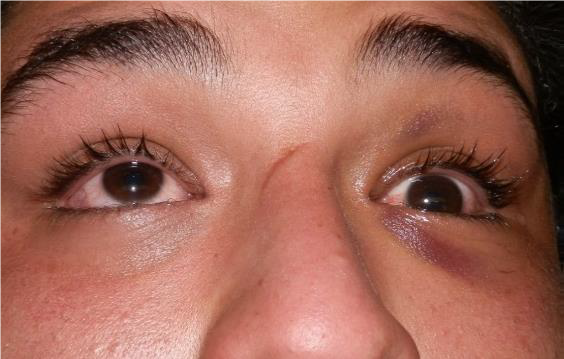
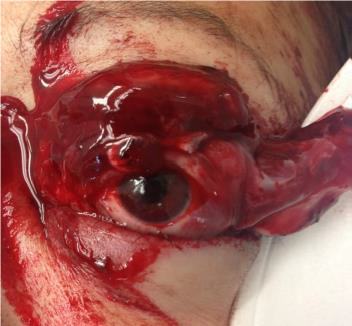
EYELID LACERATION
EXAM
Note if laceration involves eyelid margin or nasolacrimal system.
MANAGEMENT
- Maintain high suspicion for open globe.
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology.)
- SHIELD AND SHIP.
- Unless experienced, delay definitive repair for laceration involving eyelid margin for surgery by ophthalmologist. DO NOT repair in the presence of an open globe injury.
- DO NOT debride or discard tissue, even if the eye is severely traumatized.
Figures: Eyelid laceration.
THERMAL BURN
EXAM
- Facial burn
- Eyelid burn and eyelash loss
MANAGEMENT
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology.)
- SHIELD AND SHIP.
- If unable to close eyelids, start sterile eye ointment to prevent eye exposure.
- Give petrolatum or erythromycin ointment if available.
- Apply every 2-4 hours to eye surface.
- Aggressive fluid resuscitation may rarely lead to orbital compartment syndrome requiring lateral canthotomy and inferior cantholysis.
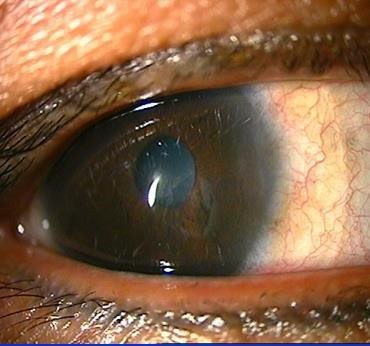
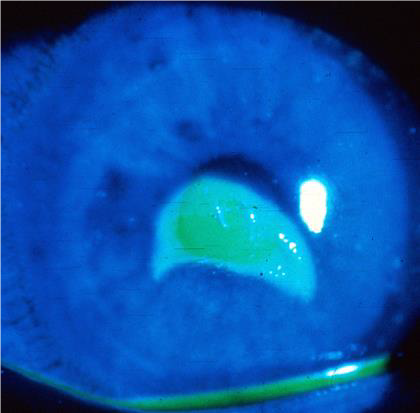
CORNEAL ABRASION
EXAM
- Epithelial defect.
- NO infiltrate (whitish opacity in cornea). Infiltrates indicate infection or inflammation of cornea.
- Stains with fluorescein when illuminated with cobalt blue light if available.
MANAGEMENT
- Ophthalmic antibiotic ointment or drops.
- Erythromycin, bacitracin, or bacitracin/polymyxin one ribbon every 2-4 hours if available OR
- Fluoroquinolone (e.g., moxifloxacin, gatifloxacin, levofloxacin) OR polymyxin B/trimethoprim instill one drop 4 times daily if available.
- Stop contact lens wear.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology.)
Figures: Corneal abrasion.
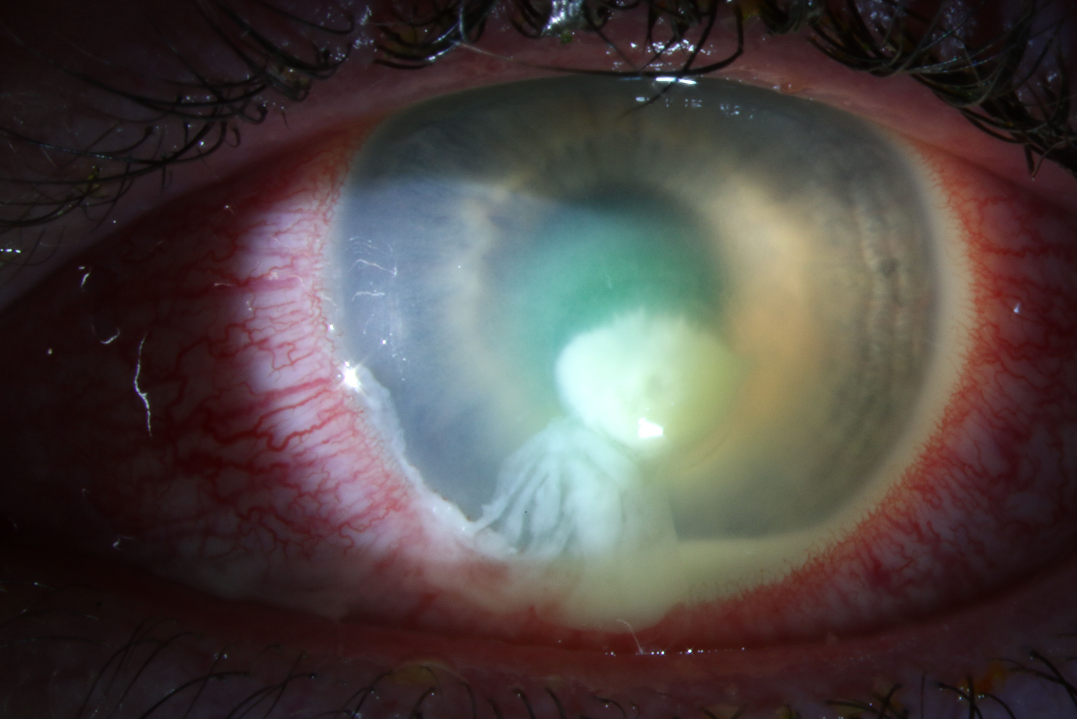
INFECTIOUS KERATITIS/CORNEAL ULCER
These conditions are commonly associated with contact lens wear or overuse. Numerous regulations prohibit or limit wear during deployment, but contact lens use during deployment continues to be common.
EXAM
- Epithelial defect
- Corneal infiltrate (whitish opacity in cornea)
MANAGEMENT
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology).
- SHIELD AND SHIP.
- Stop contact lens wear.
- Start topical antibiotic drops if available. Instill one drop every hour while awake. Give fluoroquinolone (e.g., moxifloxacin, gatifloxacin, levofloxacin) OR polymyxin B/trimethoprim if available.
CORNEAL AND CONJUNCTIVAL FOREIGN BODIES
EXAM
- Corneal or conjunctival foreign body
- If metallic, there may be a rust ring
MANAGEMENT
- Superficial foreign bodies may be irrigated away or removed with a moistened CTA under topical anesthesia (proparacaine or tetracaine drops).
- Start topical ophthalmic antibiotic drops or ointments if available.
- DO NOT remove impaled or resistant foreign bodies.
- Assess and document vision if possible.
- Teleophthalmology consultation with eye surgeon.
- SHIELD AND SHIP.
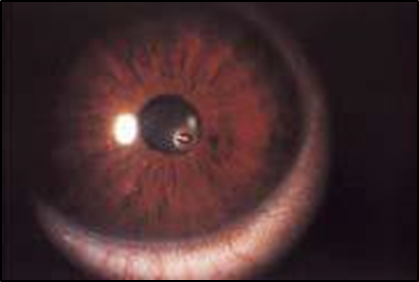
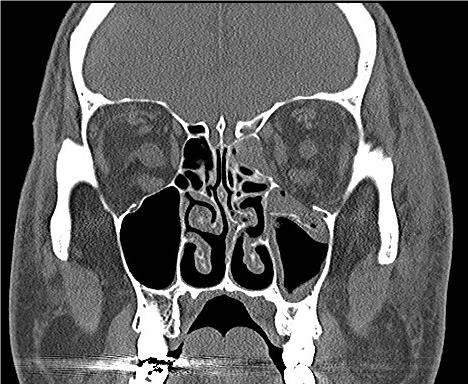
INTRAORBITAL FOREIGN BODY
EXAM
Intraorbital foreign body visible on examination or identified on diagnostic imaging such as X-ray or CT.
MANAGEMENT
- DO NOT remove impaled or resistant foreign bodies.
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology).
- SHIELD AND SHIP.
LASER EXPOSURES
- Assess and document vision.
- Report incident to supervisor.
- Follow the recommendations outlined in JTS CPG Ocular Evaluation and Disposition after Suspected Laser Exposure.49
LASIK FLAP DISLOCATION
- DO NOT amputate.
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology).
- SHIELD AND SHIP.
TRAUMATIC OPTIC NEUROPATHY
History
Vision loss after eye trauma
Exam
- Decreased visual acuity
- Afferent pupillary defect
Management
- Assess and document vision and afferent pupillary defect.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology.)
- SHIELD AND SHIP.
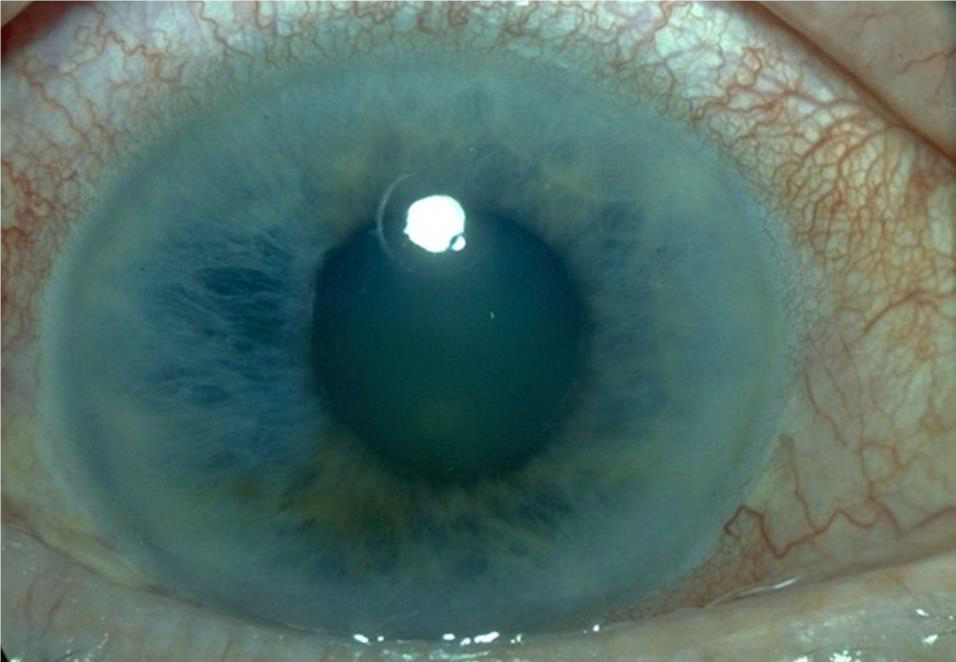
ANGLE-CLOSURE GLAUCOMA
History
Blurred vision or loss of vision, halos around lights, severe eye pain, severe headache, eye redness, nausea and vomiting.
Exam
- Palpate eyelids to see if involved eye has increased firmness compared with the opposite eye.
- Fixed, mid-dilated pupil.
- Shallow anterior chamber.
- Corneal edema (hazy cornea).
- Conjunctival injection.
Management
- Assess and document vision if possible.
- Teleophthalmology consultation with eye surgeon. (See Teleophthalmology.)
- Give acetazolamide 500 mg PO initial dose, then 250 mg PO every 4 hours to decrease IOP if available.
- Start intra ocular pressure (IOP)-lowering topical medications if available. Instill 1 drop of timolol, brimonidine, and dorzolamide 5 minutes apart. Repeat for 3 rounds.
- SHIELD AND SHIP.
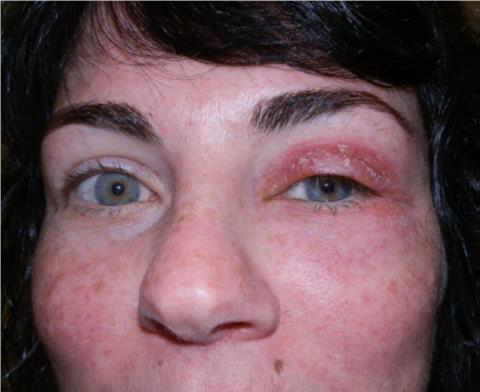
PRESEPTAL CELLULITIS
EXAM
- Eyelid redness, swelling, tenderness, and warmth.
- Usually little or no swelling or redness of conjunctiva.
- NO signs orbital involvement including restriction of eye movement, pain with eye movement, or proptosis (eye bulging forward), loss of vision, or afferent pupillary defect.
MANAGEMENT
- Assess and document vision if possible.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology).
- Start oral antibiotics Clindamycin 300 mg PO q8hrs OR Amoxicillin-clavulanic acid 875 mg PO q12hrs if available.
- SHIELD AND SHIP.
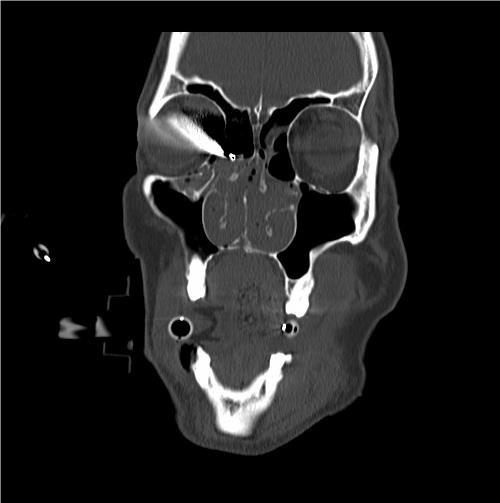
ORBITAL CELLULITIS
EXAM
- Eyelid redness, swelling, tenderness, and warmth.
- Swelling and redness of conjunctiva.
- Signs orbital involvement may include restriction of eye movement, pain with eye movement, or proptosis (eye bulging forward), loss of vision, or afferent pupillary defect.
MANAGEMENT
- Assess and document vision if possible.
- Obtain urgent CT head and orbits as soon as possible to determine if there is an abscess that needs to be surgically drained.
- Seek teleophthalmology consultation with eye surgeon. (See Teleophthalmology.)
- Start broad-spectrum IV antibiotics if available.50
- Ampicillin-sulbactam 3 g IV q6 hours OR piperacillin-tazobactam 4.5 g IV q6 hours PLUS
- Vancomycin 15-20 mg/kg IV per dose q8-12 hours, maximum 2 g for each dose.
- SHIELD AND SHIP.
PROTECTIVE EYEWEAR
- Prevent eye injuries.
- Encourage wearing only approved eye protection – eyepro or eye-armor.51-53
- MCEP
- BPE
- Discourage contact lens use.
- Encourage wearing only approved eye protection – eyepro or eye-armor.51-53
- Look for the “APEL” label.
- Army’s Program Executive Office – Soldier (PEO-Soldier) certifies all MCEP items to meet or exceed safety and ballistic fragmentation standards.
- Approved eyewear found on the APEL, available at
- http://www.peosoldier.army.mil/equipment/eyewear/
- APEL eyewear is identifiable by a neon green “APEL” logo required to be on all MCEP packaging and the “APEL” name permanently marked on the frame (located on left temple or strap).
- APEL eyepro comes in spectacle and goggle designs and in various sizes, with optical inserts available for some models.
- Service-specific policies may influence or limit the availability and variety of APEL eyepro.
- APEL eyewear should NOT be assumed to be sufficient for occupational tasks where specific safety eyewear is required (welding, chemical protection, splash protection, or other OSHA-specified tasks).
- Many commercial manufacturers produce “MIL-SPEC” polycarbonate eyewear, but only a few models pass the rigorous additional testing requirements.
- Many candidate models fail because of frame failure rather than lens failure.
- CAUTION: “Meets or exceeds MIL-SPEC” does not mean the model has passed APEL testing.
- Be particularly vigilant and cautious when ordering eyewear or replacement eyepro online.
- Counterfeit eyepro has surfaced in theater; beware of “bargain” eyepro. When purchasing eyepro, look for the official “APEL” marks and tags.
Figures 27, 28, 29: APEL labeling. U.S. Army Photo by PEO Soldier.



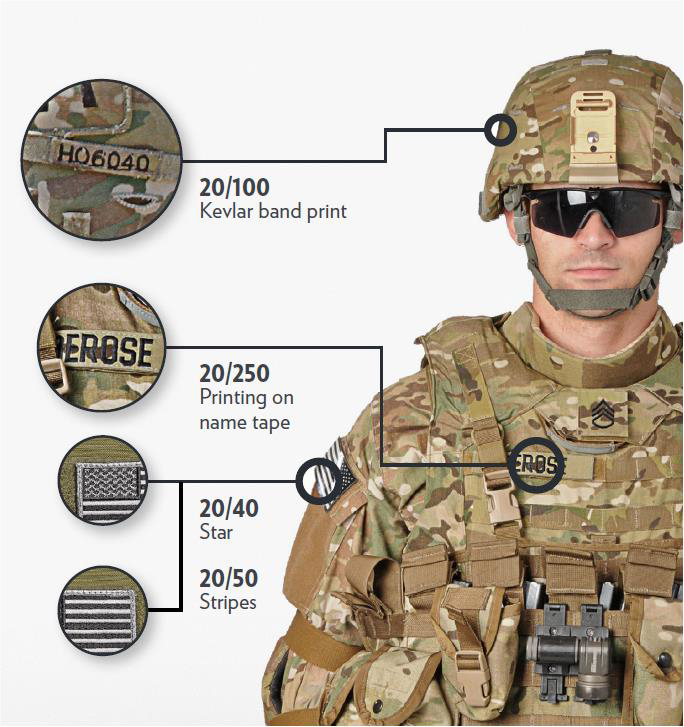
VISUAL ACUITY TESTING
- Some patients may require drop of topical anesthetic to tolerate testing.
- Decide upon testing method.
- Place the patient at the designated testing distance.
- Use Snellen eye chart if available. Start testing at 20 feet. Move patient closer if unable to see letters. Can move patient as close as 3 feet to chart.
- Letters and patches on the uniform can be used in the field. (See Figure 30 below.) Testing distance is 3 feet .54
- If patient is unable to see letters or patches, use the testing methods below:
- Count fingers: Hold up one hand, extend one or more fingers and ask the patient to count the number of fingers. Record the greatest distance from the patient at which counting fingers is done accurately.
- Hand motion: Hold up one hand and wave one hand. Record the greatest distance from the patient at which the patient can detect movement of your hand.
- Light perception: Use a penlight or other bright light source and shine in the eye. Record the patient’s response as light perception if able to detect the light. Record the patient’s response as no light perception if unable to detect the light. Record the light source used for testing (e.g. penlight or flashlight).
- Test with patient wearing glasses if available.
- Record whether testing completed with or without glasses correction.
- Test the right eye first by convention. Completely cover the left eye using the palm of your hand.
- DO NOT put pressure on eye with suspected open globe injury.
- If testing using letters, ask the patient to read the smallest line in which he or she can distinguish more than half the letters.
- Record the acuity measurement as a notation (example 20/20) in which the numerator represents the distance at which the test is performed and the denominator represents the numeric designation for the line read.
- Repeat the procedure for the other eye.
- Record visual acuity for both eyes.
- Example # 1
- Right eye: uniform name tape 3 feet without correction.
- Left eye: count fingers 2 feet without correction.
- Example # 2
- Right eye: hand motion 2 feet with glasses.
- Left eye: stripes U.S. Flag patch 3 feet with glasses.
RAPD TESTING
- Useful in the evaluation of unilateral or asymmetric vision loss, even in an unconscious patient.
- “Swinging flashlight test” technique.
- Use bright light source such as penlight or flashlight for testing.
- If patient is able to follow commands, have patient fixate on a distant object.
- Shine light into one eye and note the pupillary response.
- Swing the light quickly to the opposite eye and note the pupillary response.
- Swing the light quickly back to the first eye while maintaining the same distance from each eye.
- Normal response: Pupils constrict simultaneously and equally in response to light in either eye (consensual light reflex).
- Abnormal response: When swinging light to the injured or diseased eye, both pupils constrict less or dilate (positive relative afferent pupillary defect or Marcus Gunn pupil).
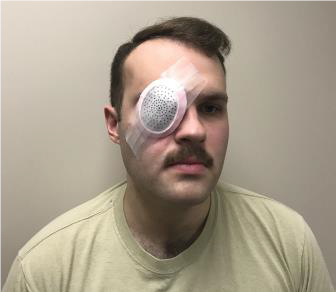
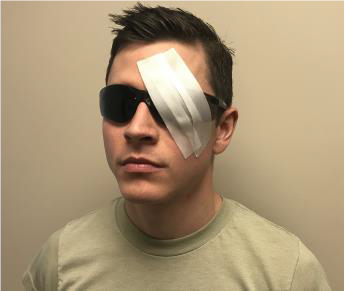
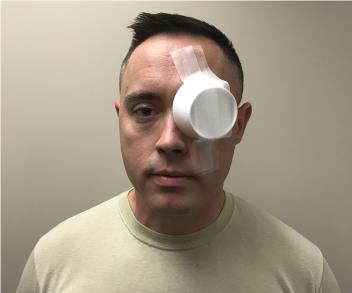
HOW TO PLACE EYESHIELD
- Use rigid eye shield.
- Fox shield if available. (See Figure 31a.)
- Can use member’s own eye protection if intact or minor damage. (See Figure 31b.)
- Other rigid materials that do not place pressure on the eye can be used as an eye shield - like a cup. (See Figure 31c.)
- Position shield so rim of the orbit supports it when taped.
- Secure shield with tape. Recommend 3 strips or enough to secure.
- Shield ONLY injured eye. Shielding both eyes increases patient anxiety.
- DO NOT patch. It puts pressure on eye. (DO shield but DO NOT patch).
- DO NOT wrap. It puts pressure on eye.
- DO NOT place anything under an eye shield including gauze.
Figure 31. Eye shields.
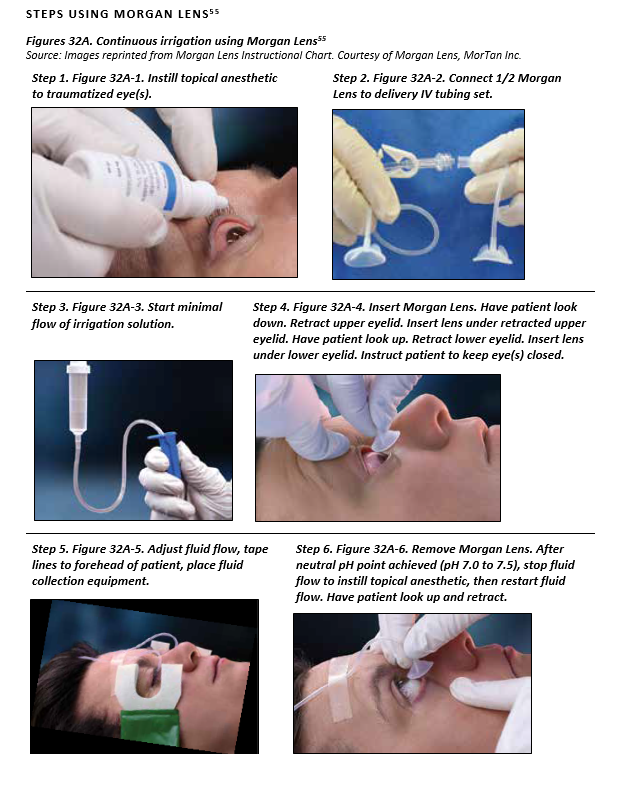
CONTINUOUS IRRIGATION USING MORGAN LENS
SUPPLIES NEEDED USING MORGAN LENS
- Topical anesthetic ( 0.5% Proparacaine or 0.5% Tetracaine)
- Morgan Lens
- IV tubing set and IV pole
- Irrigating solution – best solution choice is normal saline or lactated Ringer’s
- Fluid collection equipment (basins, bowels, or MorTan Medi-Ducts)
- pH paper or urine dipstick strips
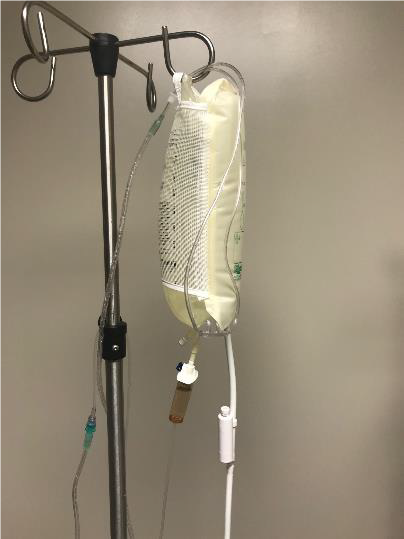
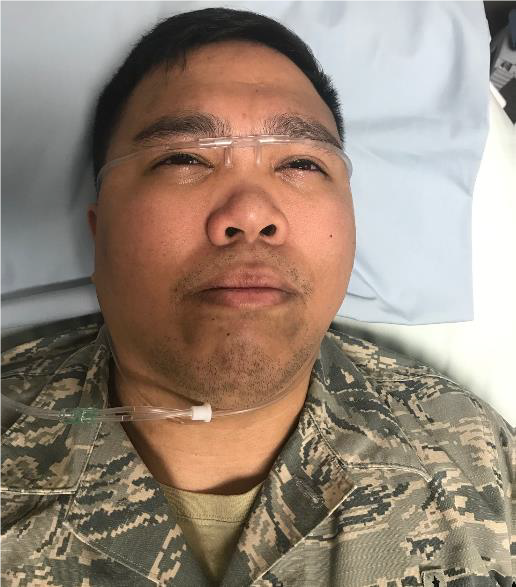
CONTINUOUS IRRIGATION USING NASAL CANNULA
SUPPLES NEEDED
- Topical anesthetic ( 0.5% Proparacaine or 0.5% Tetracaine)
- Irrigating solution – best solution choice is normal saline or lactated Ringer’s
- IV pole
- Pressure infuser
- IV tubing
- Nasal cannula
- Connection between IV tubing and nasal cannula tubing. (See Figure 32B-2).
- Tubing connector
- Stopcock connector
- Syringe tip cap (cut off top with scissors)
- Irrigating solution – best solution choice is normal saline or lactated Ringer’s.
- Fluid collection equipment (basins, bowels, or MorTan Medi-Ducts)
- pH paper or urine dipstick strips
Steps
- Instill topical anesthetic to traumatized eye(s)
- Hang IV fluid on IV pole inside pressure infuser.
- Spike and prime IV tubing.
- Connect IV tubing to nasal cannula tubing.
- Position nasal cannula on forehead so prongs provide continuous irrigation into both eyes.
- Run continuous irrigation wide open until neutral pH point achieved (pH 7.0 to 7.5)
Figure 32a-c. Continuous irrigation for chemical injury using nasal cannula.

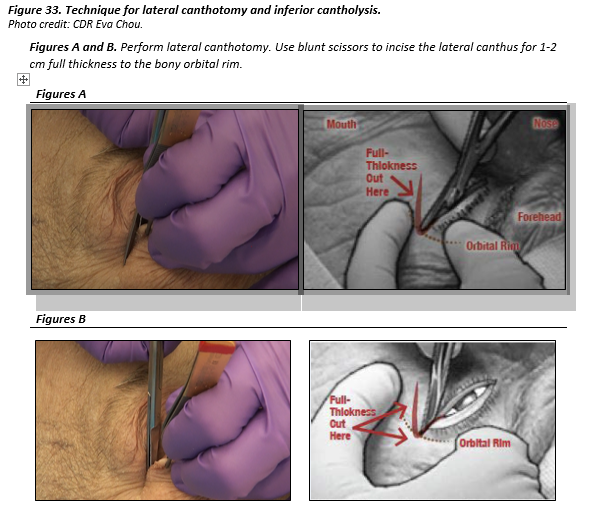
LATERAL CANTHOTOMY AND INFERIOR CANTHOLYSIS
- If possible, give subdermal injection of lidocaine 2% with epinephrine to the lateral canthus. It can be difficult to inject tissue that is already swollen.
- Optional only if good local anesthesia. Apply a hemostat or needle driver horizontally at the lateral canthus for 1 minute to reduce bleeding.
- Perform lateral canthotomy. Use blunt scissors to incise the lateral canthus, FULL THICKNESS for 1-2 cm to the lateral bony orbital rim (canthotomy).
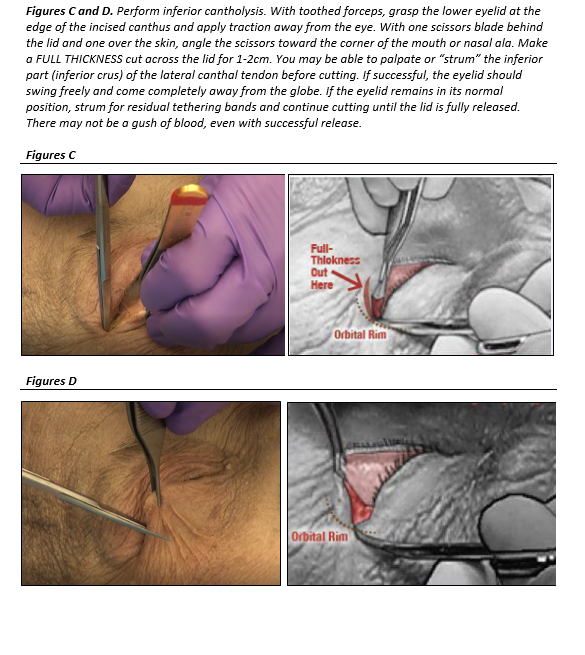
- Perform inferior cantholysis:
- With toothed forceps, grasp the lower eyelid firmly at the edge of the incised canthus (at the orbital rim) and apply traction away from the eye.
- Keeping one scissor blade behind the lid (in the conjunctival fornix) and one blade over the skin, angle the scissors toward the corner of the mouth or nasal ala. Make a FULL THICKNESS cut across the lower lid for length of 1-2cm. This should fully disinsert the lid. You may be able to palpate or “strum” the inferior part (inferior crus) of the lateral canthal tendon as a “guitar string” before cutting.
- If successful, the eyelid should swing freely and come completely away from the globe. If the eyelid remains in its normal position, use scissors tips to feel for residual tethering bands, and continue cutting until the lid swings freely.
- There may not be a “gush of blood” even with successful release.
- Recheck vision and intraocular pressure. If the intraocular pressure remains elevated with poor vision and a tense orbit, then perform superior cantholysis, separating the upper lid from the orbital margin. Also consider giving 500MG IV acetazolamide.
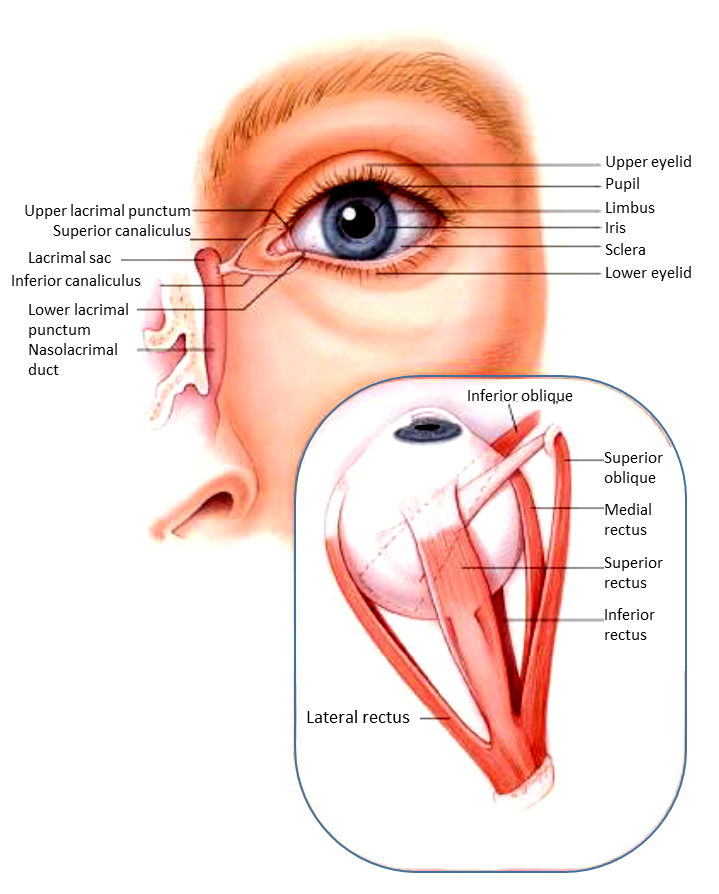
Eye Anatomy
AEROMEDICAL EVACUATION CONSIDERATIONS
Due to the requirement to move the coalition patient with vision-threatening injuries to a Role 3 with an ophthalmologist in theater or Role 4 outside the area of responsibility (AOR), early and safe transport of these patients should involve consideration of several factors.
- Initiate teleophthalmology consultation with an ophthalmologist as soon as possible.
- Management of concomitant injuries is crucial during flight.
- After open globe injury, rotary wing aeromedical evacuation may be accomplished in theater without prior clearance by an ophthalmologist.
- Teleophthalmology consultation with an ophthalmologist is recommended before fixed wing transport of an open globe injury. Typically the patient is cleared for immediate transport. If available, CT imaging of the orbit with thin slices can identify if there is trapped intraocular air, which could cause an acute rise in intraocular pressure or extrusion of intraocular contents in a depressurized cabin. In this situation, the aircraft commander should be notified that the internal cabin pressure should be maintained between sea level and 2500 feet to keep gas expansion at or below 10%. The aircraft commander can determine a safe aircraft altitude for that mission based on that information. For example, if the transport aircraft is a C-17, cabin altitude can be up to 2500 feet and allow the aircraft to fly safely at 24,000 feet.
- Vibration and turbulence can also be increased during flight. Adequate immobilization of foreign bodies to prevent further injury. Pain related to traumatic injuries is exacerbated by vibration and adequate pain medication is required.
- Decreased visual input will increase the risk of motion sickness. Vomiting can increase the risk of further injury. Anti-emetics can help to mitigate this risk.
- The need to Valsalva or perform similar maneuvers to equalize middle ear pressure can further injure the eye and surrounding structures, particularly the inferior orbit which overlies the maxillary sinuses. Adequate rapid acting decongestants (i.e. Afrin) can be used.
- Air handling systems on board the aircraft result in a very low relative humidity. Generous use of medications to lubricate the eye may be necessary.
- Patients with eye injuries cannot generally safely navigate inside aircraft due to poor lighting and cramped space and decrease in visual cues to balance. Consider placing the patient on a litter for safety and a non-medical attendant to help patient move if needed.
PERFORMANCE IMPROVEMENT (PI) MONITORING
Population of Interest
- All patients with diagnosis of eye injury.
- All patients with a diagnosis of retrobulbar hematoma or orbital compartment syndrome.
INTENT (EXPECTED OUTCOMES)
- All patients in populations of interest have visual acuity tested and documented in both eyes at all roles of care (including point of injury). If the patient is unresponsive or intubated and sedated this is documented and the pupils are checked for a relative afferent pupillary defect.
- All patients in populations of interest have a rigid eye shield placed correctly and documented at all roles of care (including point of injury) that do not have an ophthalmologist.
- All patients with open globe injuries receive appropriate antibiotics at the first role of care.
- All patients with a diagnosis of retrobulbar hematoma or orbital compartment syndrome receive an immediate full thickness lateral canthotomy and inferior cantholysis.
- All patients in populations of interest receive a teleophthalmology consultation.
- All patients in population of interest with vision-threatening injuries are evacuated so that they are able to receive treatment by an ophthalmologist within 24 hours. If evacuation is not possible, the reason for the delay is documented.
PERFORMANCE/ADHERENCE MEASURES
- Number and percentage of patients in the populations of interest that have visual acuity tested and documented in both eyes.
- Number and percentage of patients in the populations of interest who have rigid eye shield placed correctly and documented at all roles of care (including point of injury) that do not have an ophthalmologist.
- Number and percentage of patients with open globe injury who receive appropriate antibiotic at the first role of care.
- Number and percentage of patients with a diagnosis of retrobulbar hematoma or orbital compartment syndrome who receive an immediate full thickness lateral canthotomy and inferior cantholysis.
- Number and percentage of patients in the populations of interest who receive a teleophthalmology consultation.
- Number and percentage of vision-threatening injuries that are evacuated so that they are able to receive treatment by an ophthalmologist within 24 hours. If evacuation not possible, the reason for the delay is documented.
DATA SOURCES
- Patient Record
- Department of Defense Trauma Registry (DoDTR)
SYSTEM REPORTING & FREQUENCY
The above constitutes the minimum criteria for PI monitoring of this CPG. System reporting will be performed annually; additional PI monitoring and system reporting may be performed as needed.
The system review and data analysis will be performed by the Joint Trauma System (JTS) Chief, JTS Program Manager, and the JTS PI Branch.
RESPONSIBILITIES
It is the trauma team leader’s responsibility to ensure familiarity, appropriate compliance and PI monitoring at the local level with this CPG.
REFERENCES
- Mader TH, Aragones JV, Chandler AC, et al. Ocular and ocular adnexal injuries treated by United States military ophthalmologists during Operations Desert Shield and Desert Storm. Ophthalmology. 1993 Oct;100(10):1462-7.
- Thach AB, Johnson AJ, Carroll RB, et al. Severe eye injuries in the war in Iraq, 2003–2005. Ophthalmology. 2008 Feb;115(2):377–82.
- Blanch RJ, Bindra MS, Jacks AS, Scott RA. Ophthalmic injuries in British Armed Forces in Iraq and Afghanistan. Eye (Lond). 2011;25(2):218-23.
- Office of the Surgeon General, Department of the Army. Combat casualty care: Lessons learned from OEF and OIF, Chapter 7 Ocular Trauma, Borden Institute; 2012: 299-342.
- Weichel ED, Colyer MH. Combat ocular trauma and systemic injury. Curr Opin Ophthalmol. 2008 Nov;19(6):519-25.
- Johnson AJ. Eye trauma management: OIF clinical considerations. J Trauma 2007; 62(6 Suppl):S20–S21.
- Cho RI, Bakken HE, Reynolds ME, et al. Concomitant cranial and ocular combat injuries during Operation Iraqi Freedom. J Trauma 2009 Sep;67(3):516-20.
- Allen RC, Harper RA, Eds. Basic Ophthalmology: Essentials for medical students. 10th ed. San Francisco, CA: American Academy of Ophthalmology; 2017.
- Greven CM, Engelbrecht NE, Slusher MM, Nagy SS. Intraocular foreign bodies: management, prognostic factors, and visual outcomes. Ophthalmology. 2000 Mar;107(3):608-12.
- Office of the Surgeon General, Department of the Army, Thach AB, ed. Ophthalmic care of the combat casualty; Textbook of Military Medicine. Borden Institute; 2003.
- Office of the Surgeon General, Department of Army. Emergency War Surgery, Ocular Injuries. 5thS. Edition. Chap 14. Borden Institute; 2018: 199-212.
- Mines MJ, Bower KS, Lappan CM, et al. The United States Army Ocular Teleconsultation program 2004 through 2009. Am J Ophthalmol. 2011 Jul;152(1):126-132.e2.
- Colyer MH, Chun DW, Bower KS, Dick JS, Weichel ED. Perforating globe injuries during operation Iraqi Freedom. Ophthalmology. 2008 Nov;115(1):2087-93.
- Gerstenblith AT, Rabinowitz MP, eds. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 7th ed. Philadelphia: Lippincott; 1994.
- Lieb DF, Scott IU, Flynn HW Jr, Miller D, Feuer WJ. Open globe injuries with positive intraocular cultures: factors influencing final visual acuity outcomes. Ophthalmology. 2003 Aug;110(8):1560-6.
- Ahmed Y, Schimel AM, Pathengay A, et al. Endophthalmitis following open-globe injuries. Eye (Lond). 2012 Feb;26(2):212-7.
- Miller JW ed. Benchmark protocols for managing eye trauma. Eye Insights Issue 1: Ocular Trauma.
- Andreoli CM, Andreoli MT, Kloek CE, et al. Low rate of endophthalmitis in a large series of open globe injuries. Am J Ophthalmol. 2009 Apr;147(4):601-608.e2.
- US Eye Injury Registry.
- Bhagat N, Nagori S, Zarbin M. Post-traumatic Infectious Endophthalmitis. Surv Ophthalmol. 2011 May-Jun;56(3):214-51.
- Kuhn F, Pieramici D, eds. Ocular trauma: Principles and practice. New York, NY: Thieme; 2002: 293-300.
- Essex RW, Yi Q, Charles PG, Allen PJ. Post-traumatic endophthalmitis. Ophthalmology. 2004 Nov;111(11):2015-22.
- Zhang Y, Zhang MN, Jiang CH, Yao Y, Zhang K. Endophthalmitis following open globe injury. Br J Ophthalmol. 2010 Jan;94(1):111-4.
- Schmidseder E, Miño de Kaspar H, Klauss V, Kampik A. Post-traumatic endophthalmitis after penetrating eye injuries. Risk factors, microbiological diagnosis and functional outcome. Ophthalmologe. 1998 Mar;95(3):153-7.
- Blanch RJ, Bishop J, Javidi H, Murray PI. Effect of time to primary repair on final visual outcome after open globe injury. Br J Ophthalmol. 2019 Jan 12.
- Oztürk F, Kortunay S, Kurt E, et al Penetration of topical and oral ciprofloxacin into the aqueous and vitreous humor in inflamed eyes. Retina. 1999;19(3):218-22.
- Oztürk F, Kortunay S, Kurt E, et al. Effects of trauma and infection on ciprofloxacin levels in the vitreous cavity. Retina. 1999;19(2):127-30.
- Hariprasad SM, Mieler WF, Holz ER. Vitreous and aqueous penetration of orally administered gatifloxacin in humans. Arch Ophthalmol. 2003 Mar;121(3):345-50.
- Fuller JJ, Marcus DM. Vitreous and aqueous penetration of orally administered gatifloxacin in humans. Arch Ophthalmol. 2004 Sep;122(9):1408-9; author reply 1409.
- Vedantham V, Lalitha P, Velpandian T, et al. Vitreous and aqueous penetration of orally administered moxifloxacin in humans. Eye (Lond). 2006 Nov;20(11):1273-8. Epub 2006 Sep 30.
- Al-Omran AM, Abboud EB, Abu El-Asrar AM . Microbiologic spectrum and visual outcome of posttraumatic endophthalmitis. Retina. 2007;27(2):236.
- Duch-Samper AM, Chaqués-Alepuz V, Menezo JL, et al. Endophthalmitis following open-globe injuries. Curr Opin Ophthalmol. 1998;9(3):59.
- Affeldt JC, Flynn HW Jr, Forster RK, et al. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology. 1987;94(4):407.
- Soheilian M, Rafati N, Mohebbi MR, et al. Prophylaxis of acute posttraumatic bacterial endophthalmitis: a multicenter, randomized clinical trial of intraocular antibiotic injection, report 2. Arch Ophthalmol. 2007 Apr;125(4):460-5.
- Wadia S, Bhola R, Lorenz D, et al Ketamine and intraocular pressure in children. Ann Emerg Med. 2014 Oct;64(4):385-388.
- Halstead SM, Deakyne SJ, Bajaj L, et al. The effect of ketamine on intraocular pressure in pediatric patients during procedural sedation. Acad Emerg Med. 2012 Oct;19(10):1145-50.
- Ehlers JP, Kunimoto DY, Ittoop S, et al. Metallic intraocular foreign bodies: characteristics, interventions, and prognostic factors for visual outcome and globe survival. Am J Ophthalmol. 2008 Sep;146(3):427-433.
- Woodcock MG, Scott RA, Huntbach J, Kirkby GR. Mass and shape as factors in intraocular foreign body injuries. Ophthalmology. 2006 Dec;113(12):2262-9.
- Yang CS, Lu CK, Lee FL, et al. Treatment and outcome of traumatic endophthalmitis in open globe injury with retained intraocular foreign body. Ophthalmologica. 2010;224(2):79-85.
- Colyer MH, Weber ED, Weichel ED, et al. Delayed intraocular foreign body removal without endophthalmitis during OIF and OEF. Ophthalmology 2007 Aug; 114(8):1439–47.
- Jonas JB, Knorr HL, Budde WM. Prognostic factors in ocular injuries caused by intraocular or retrobulbar foreign bodies. Ophthalmology. 2000 May;107(5):823-8.
- Thompson JT, Parver LM, Enger CL, et al Infectious endophthalmitis after penetrating injuries with retained intraocular foreign bodies. National Eye Trauma System. Ophthalmology. 1993 Oct;100(10):1468-74.
- Jonas JB, Budde WM. Early versus late removal of retained intraocular foreign bodies. Retina. 1999;19(3):193-7.
- Ferrari TM, Cardascia N, Di Gesù I, et al. Early versus late removal of retained intraocular foreign bodies. Retina. 2001;21(1):92-3.
- Cockerham GC, Rice TA, Hewes EH, et al. Closed-eye ocular injuries in the Iraq and Afghanistan wars. N Engl J Med. 2011 Jun 2;364(22):2172-3.
- Weichel ED, Colyer MH, Ludlow SE, et al. Combat ocular trauma visual outcomes during operations Iraqi and enduring freedom. Ophthalmology. 2008 Dec;115(12):2235-45.
- Harris MD, Lincoln AE, Amoroso PJ, et al. Laser eye injuries in military occupations. Aviat Space Environ Med. 2003 Sep;74(9):947-52.
- Gooch JM, Harvey RR, Parham-Bruce W, el al. U.S. Air Force School of Aerospace Medicine Laser Injury Guidebook, Apr 2012.
- Brown J Jr, Hacker H, Schuschereba ST, et al. Steroidal and nonsteroidal anti-inflammatory medications can improve photoreceptor survival after laser retinal photocoagulation. Ophthalmology 2007 Oct; 114(10):1876-83.
- Howe L, Jones NS. Guidelines for the management of periorbital cellulitis/abscess. Clin Otolaryngol Allied Sci. 2004 Dec;29(6):725-8.
- Thomas R, McManus JG, Johnson A, et al. Ocular injury reduction from ocular protection use in current combat operations. J Trauma. 2009 Apr;66(4 Suppl):S99-103.
- Gondusky JS, Reiter MP. Protecting military convoys in Iraq: an examination of battle injuries sustained by a mechanized battalion during Operation Iraqi Freedom II. Mil Med. 2005 Jun;170(6):546-9.
- Breeze J, Allanson-Bailey LS, Hunt NC, et al. Surface wound mapping of battlefield occulo-facial injury. Injury. 2012 Nov;43(11):1856-60.
- Godbole NJ, Seefeldt ES, Raymond WR, et al. Simplified method for rapid field assessment of visual acuity by first responders after ocular injury. Mil Med. 2018 Mar 1;183(suppl_1):219-223.
- (2018). Resources: Morgan lens power-point presentation. Retrieved from https://www.morganlens.com/resource-library.
APPENDIX A: ADDITIONAL INFORMATION REGARDING OFF-LABEL USES IN CPGS
PURPOSE
The purpose of this Appendix is to ensure an understanding of DoD policy and practice regarding inclusion in CPGs of “off-label” uses of U.S. Food and Drug Administration (FDA)–approved products. This applies to off-label uses with patients who are armed forces members.
BACKGROUND
Unapproved (i.e. “off-label”) uses of FDA-approved products are extremely common in American medicine and are usually not subject to any special regulations. However, under Federal law, in some circumstances, unapproved uses of approved drugs are subject to FDA regulations governing “investigational new drugs.” These circumstances include such uses as part of clinical trials, and in the military context, command required, unapproved uses. Some command requested unapproved uses may also be subject to special regulations.
ADDITIONAL INFORMATION REGARDING OFF-LABEL USES IN CPGS
The inclusion in CPGs of off-label uses is not a clinical trial, nor is it a command request or requirement. Further, it does not imply that the Military Health System requires that use by DoD health care practitioners or considers it to be the “standard of care.” Rather, the inclusion in CPGs of off-label uses is to inform the clinical judgment of the responsible health care practitioner by providing information regarding potential risks and benefits of treatment alternatives. The decision is for the clinical judgment of the responsible health care practitioner within the practitioner-patient relationship.
ADDITIONAL PROCEDURES
Balanced Discussion
Consistent with this purpose, CPG discussions of off-label uses specifically state that they are uses not approved by the FDA. Further, such discussions are balanced in the presentation of appropriate clinical study data, including any such data that suggest caution in the use of the product and specifically including any FDA-issued warnings.
Quality Assurance Monitoring
With respect to such off-label uses, DoD procedure is to maintain a regular system of quality assurance monitoring of outcomes and known potential adverse events. For this reason, the importance of accurate clinical records is underscored.
Information to Patients
Good clinical practice includes the provision of appropriate information to patients. Each CPG discussing an unusual off-label use will address the issue of information to patients. When practicable, consideration will be given to including in an appendix an appropriate information sheet for distribution to patients, whether before or after use of the product. Information to patients should address in plain language: a) that the use is not approved by the FDA; b) the reasons why a DoD health care practitioner would decide to use the product for this purpose; and c) the potential risks associated with such use.
DOWNLOAD CPG TO PDF

READ FULL PDF

READ FULL PDF

READ FULL PDF



