APPENDIX B: MEDICAL COUNTERMEASURE TABLES
Disclaimer: This appendix may or may not discuss investigational, unapproved, or off-label use of drugs or devices. It is based upon the best information available at the time of publication. Patients and physicians are advised to consult prescribing information for products discussed in this appendix. The information provided in this appendix is not meant to substitute for the independent clinical judgment of a physician, relative to diagnostic or treatment options for a specific patient’s medical condition. It is designed to provide information and assist decision making. It is not intended to define a standard of care and should not be construed as one; neither should it be interpreted as prescribing an exclusive course of management. This appendix was developed by experts in this field. Variations in practice will inevitably and appropriately occur when clinicians consider the needs of individual patients, available resources, and limitations unique to an institution or type of practice. Every healthcare professional making use of this appendix is responsible for evaluating the appropriateness of applying it in the setting of any clinical situation. This appendix does not supersede any DoD policy.
INFORMATION FOR OPTIMAL USE OF APPENDIX B:
These tables are designed to provide a snapshot of the pre-exposure prophylaxis (PrEP), post-exposure prophylaxis (PEP) and treatment MCMs available for select biothreats. This appendix will be updated as rapidly as possible as new threats emerge, or as new information on MCMs are developed. For additional information on vaccines, DHA maintains a website that displays vaccination recommendations by CCMD (https://www.health.mil/Military-Health-Topics/Health-Readiness/Immunization-Healthcare/Vaccine-Recommendations/Vaccine-Recommendations-by-AOR). There are nuances to utilization of many of these MCMs, so utilize the hyperlinks for references given by agent for dosing and duration. Given that FDA labels are periodically updated or generic manufacturers change, so hyperlinks to labels are not included by MCM. Either DAILYMED (https://dailymed.nlm.nih.gov/dailymed/index.cfm) or Drugs@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm ) can be utilized to find the most up-to-date product labels. DAILYMED has more user-friendly search features but note that the "in use" labeling on DailyMed may not be identical to the most recent FDA-approved labeling available at Drugs@FDA, or the labeling distributed with products. This appendix also links to additional information on potential uses of repurposed MCMs within the practitioner-patient relationship (CAC enabled website: https://pki.jacks.jpeocbrnd.army.mil/JPMCBRNMedical/Dashboard/RaidrSummaries). Finally, the U.S. Army Medical Materiel Development Activity (USAMMDA) provides a number of Force Health Protection (FHP) protocols and are noted in the tables below if available.
For more information, contact: USAMMDA FHP protocol portfolio: 301-401-2768 (24/7), Email: usarmy.detrick.medcom-usammda.mbx.force-health-protection@health.mil
1. MCMS FOR BACTERIAL AGENTS
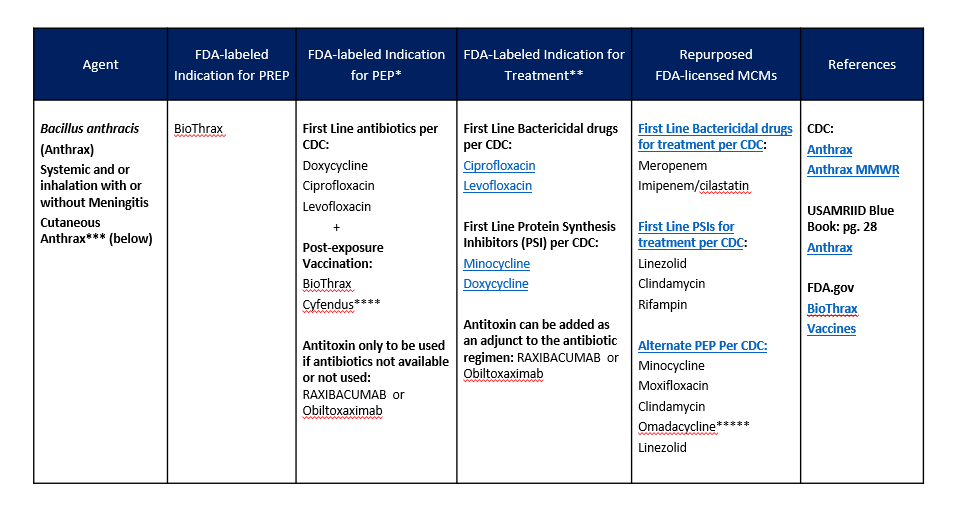

MCMS FOR BACTERIAL AGENTS (CONTINUED)
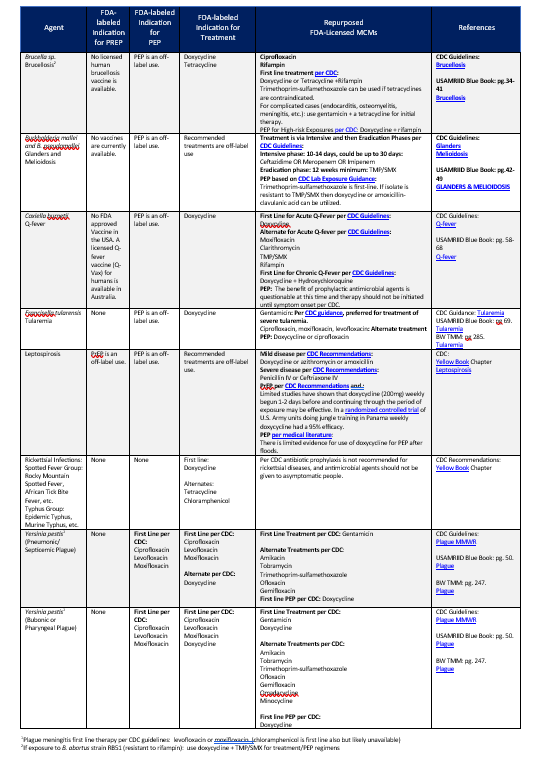
2. MCMS FOR VIRAL AGENTS
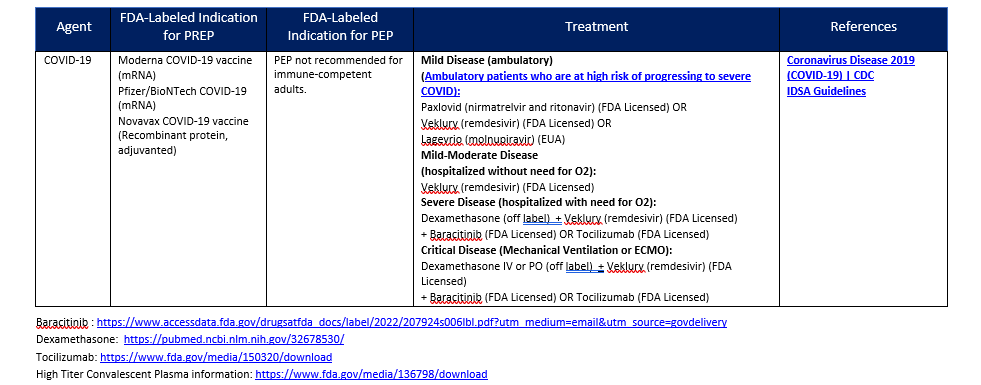
MCMS FOR VIRAL AGENTS (CONTINUED)
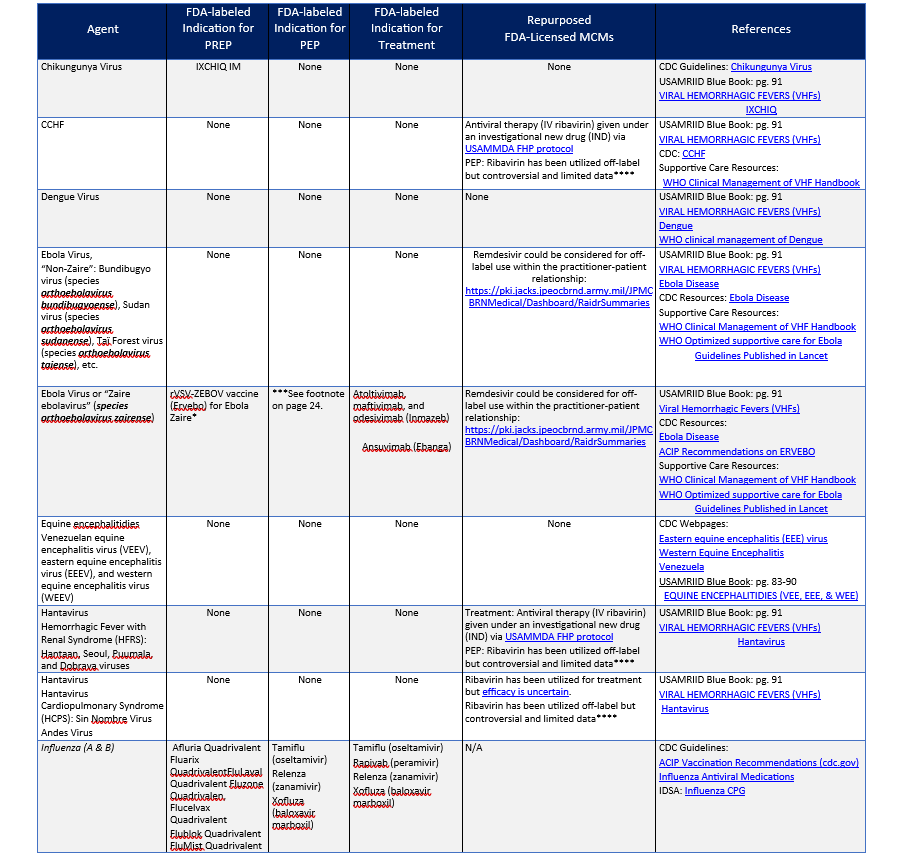
MCMS FOR VIRAL AGENTS (CONTINUED)
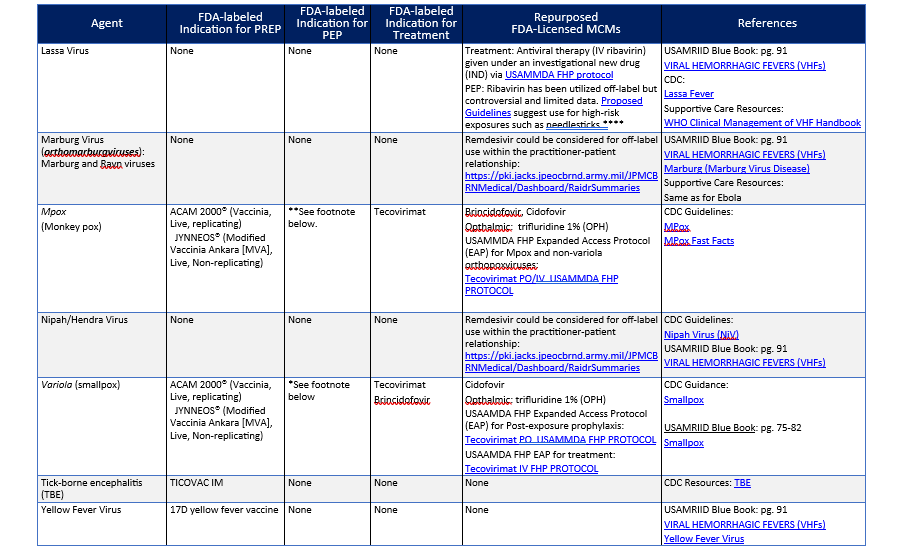
*CDC has clinical guidance for smallpox vaccine use in a post-event vaccination program. In a public health emergency involving smallpox, vaccination with replication-competent (ACAM2000) smallpox vaccine would be the optimal post-exposure vaccination strategy for stopping the chain of transmission and achieving epidemic control. This is because ACAM2000 is a replication competent vaccine that produces a rapid immune response in comparison to JYNNEOS (also known as Invamune) which requires two doses, four weeks apart to achieve a comparable immune response. Replication competent vaccinia vaccine (DRYVAX) from which ACAM2000 was derived was utilized in a ring vaccination strategy to eradicate smallpox. Optimally data from the eradication campaign shows that post-exposure vaccination less than three days after exposure is optimal for prevention but vaccination greater than three days after exposure to the virus may still decrease morbidity and mortality. For more details see the referenced CDC clinical guidance particularly for algorithms of when to utilize ACAM2000 versus JYNNEOS in relation to absolute and relative contraindications to ACAM2000.
**JYNNEOS has been the dominant vaccine utilized in the U.S. instead of ACAM2000 because of the lesser adverse event profile. For PEP vaccination, CDC guidance recommends that JYNNEOS should be given as soon as possible, ideally within four days of exposure; administration four through 14 days after exposure may still provide some protection against Mpox.
*** The FDA licensed indication for ERVEBO is for the prevention of endemic disease caused by Zaire ebolavirus. ACIP recommends ERVEBO as PrEP for those responding to an outbreak of Ebola, who work as laboratorians and support staff working at biosafety level 4 (BSL-4) or Laboratory Response Network facilities in the United States that handle specimens that contain or might contain replication-competent EBOV, or Healthcare personnel (HCP) at federally designated Ebola Treatment Centers or state-designated Special Pathogens Treatment Centers involved in the care and transport of patients infected or suspected to be infected with EBOV. ERVEBO is not planned for commercial marketing but is maintained in the Strategic National Stockpile (SNS). The CDC will provide ERVEBO when requested by licensed healthcare providers from institutions or sites with individuals who meet the eligibility criteria (see https://www.cdc.gov/vhf/ebola/clinicians/vaccine/vaccine-request.html for request instructions). CDC also has an ERVEBO IND program to administer booster doses in individuals who were previously vaccinated with ERVEBO (e.g., ≥ six months since prior vaccination) and are at potential occupational risk for exposure to Zaire ebolavirus (see https://www.cdc.gov/vhf/ebola/clinicians/vaccine/booster-request.html for request instructions). ERVEBO has efficacy when given post-exposure to contacts of cases (and contacts of contacts in a ring vaccination strategy) in endemic Zaire ebolavirus outbreaks. ERVEBO has also been administered post-exposure to health care workers who have had occupational exposure during natural outbreaks.
**** Dosage Regimens of Oral Ribavirin Recommended or Used for Post-exposure Prophylaxis Following Exposure to Hantaviruses, Lassa fever (LF), and CCHF Viruses in Adults1
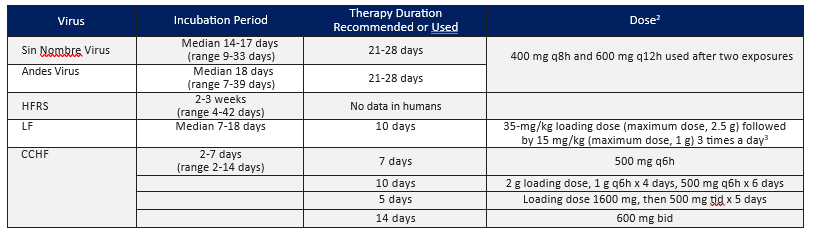
- Adapted from Rusnak, J. M. (2011). Experience with Ribavirin for Treatment and Postexposure Prophylaxis of Hemorrhagic Fever Viruses: Crimean Congo Hemorrhagic Fever, Lassa Fever, and Hantaviruses. Applied Biosafety, 16(2), 67–87. https://doi.org/10.1177/153567601101600203.
- Oral ribavirin should be started immediately after the high-risk exposure, but not before counseling of the patient by the physician. The drug should be taken with food. The patient should be informed that the efficacy of PEP is unknown and that, although there are no major risks to its use, minor adverse effects often occur. Relative contraindications to ribavirin PEP include severe anemia or hemoglobinopathy, pregnancy and breast-feeding, coronary artery disease, renal insufficiency, decompensated liver disease, and known hypersensitivity. Baseline hemoglobin and hematocrit levels should be measured, and therapy should be reconsidered if significant anemia is present. The complete blood count and bilirubin level should be rechecked 5–7 days after initiation of the drug, and ribavirin should be stopped or the dose should be adjusted if significant anemia is noted.
- Bausch DG, Hadi CM, Khan SH, Lertora JJ. Review of the literature and proposed guidelines for the use of oral ribavirin as postexposure prophylaxis for Lassa fever. Clin Infect Dis. 2010 Dec 15;51(12):1435-41.
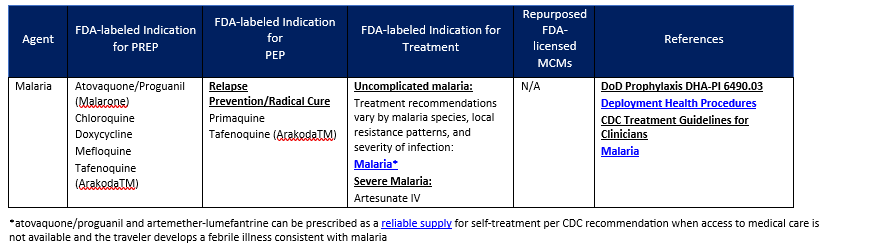
3. MCMS FOR MALARIA
4. WOUND INFECTIONS (BACTERIAL AND FUNGAL)
Relevant JTS CPGs to review:
- Infection Prevention in Combat-related Injuries
- War Wounds: Debridement and Irrigation
- Acute Traumatic Wound Management in the Prolonged Field Care Setting
- Invasive Fungal Infection in War Wounds
- Sepsis Management in Prolonged Field Care
- Prolonged Casualty Care Guidelines
- Tactical Combat Casualty Care Guidelines
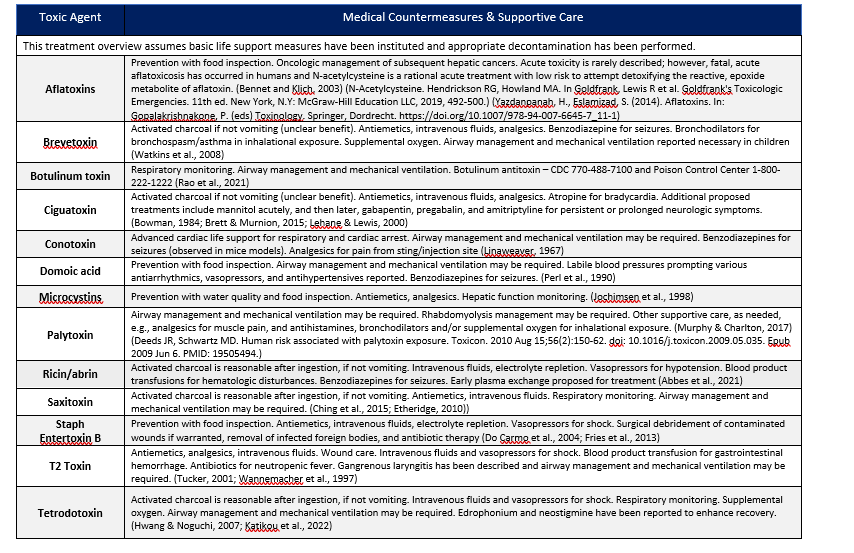
5 TOXINS ENCOUNTERED IN THE ENVIRONMENT OR ARE CONSIDERED TRADITIONAL BIOTHREATS
- Abbes M, Montana M, Curti C, Vanelle P. Ricin poisoning: A review on contamination source, diagnosis, treatment, prevention and reporting of ricin poisoning. Toxicon. 2021 May;195:86-92.
- Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003 Jul;16(3):497-516.
- Brett J, Murnion B. Pregabalin to treat ciguatera fish poisoning. Clin Toxicol (Phila). 2015 Jul;53(6):588.
- Bowman PB. Amitriptyline and ciguatera. Med J Aust. 1984 Jun 23;140(13):802. doi: 10.5694/j.1326-5377.1984.tb132642.x.
- Ching PK, Ramos RA, de los Reyes VC, Sucaldito MN, Tayag E. Lethal paralytic shellfish poisoning from consumption of green mussel broth, Western Samar, Philippines, August 2013. Western Pac Surveill Response J. 2015 May 8;6(2):22-6.
- Do Carmo LS, Cummings C, Linardi VR, et al. A case study of a massive staphylococcal food poisoning incident. Foodborne Pathog Dis. 2004 Winter;1(4):241-6.
- Etheridge SM. Paralytic shellfish poisoning: seafood safety and human health perspectives. Toxicon. 2010 Aug 15;56(2):108-22. doi: 10.1016/j.toxicon.2009.12.013.
- Fries BC, Varshney AK. Bacterial Toxins-Staphylococcal Enterotoxin B. Microbiol Spectr. 2013 Dec;1(2):10.1128/microbiolspec.AID-0002-2012. doi: 10.1128/microbiolspec.AID-0002-2012.
- Hwang DF, Noguchi T. Tetrodotoxin poisoning. Adv Food Nutr Res. 2007;52:141-236.
- Jochimsen EM, Carmichael WW, An JS, et al. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N Engl J Med. 1998 Mar 26;338(13):873-8.
- Katikou P, Gokbulut C, Kosker AR, Campàs M, Ozogul F. An updated review of tetrodotoxin and its peculiarities. Mar Drugs. 2022 Jan 3;20(1):47.
- Lehane L, Lewis RJ. Ciguatera: recent advances but the risk remains. Int J Food Microbiol. 2000 Nov 1;61(2-3):91-125. doi: 10.1016/s0168-1605(00)00382-2.
- Linaweaver PG. Toxic marine life. Mil Med. 1967 Jun;132(6):437-42. PMID: 4963183.
- Murphy LT, Charlton NP. Prevalence and characteristics of inhalational and dermal palytoxin exposures reported to the National Poison Data System in the U.S. Environ Toxicol Pharmacol. 2017 Oct;55:107-109.
- Perl TM, Bédard L, Kosatsky T, Hockin JC, Todd EC, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med. 1990 Jun 21;322(25):1775-80.
- Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical Guidelines for Diagnosis and Treatment of Botulism, 2021. MMWR Recomm Rep. 2021 May 7;70(2):1-30.
- Tucker, J. B. (2001). The “yellow rain” controversy: Lessons for arms control compliance. The Nonproliferation Review, 8(1), 25-42.
- Wannemacher R, Wiener W, Sidell SL, et al.Trichothecene mycotoxins. Medical aspects of chemical and biological warfare, 1997. 6, 655-76.
- Watkins SM, Reich A, Fleming LE, Hammond R. Neurotoxic shellfish poisoning. Mar Drugs. 2008;6(3):431-55.