Frozen and Deglycerolized Red Blood Cells
Joint Trauma System
Frozen and Deglycerolized Red Blood Cells
Summary of Changes
- Updates on current operational employment of frozen and deglycerolized red blood cells (RBCs).
- Update on quality metrics that must be met to produce deglycerolized RBCs.
- General administrative updates.
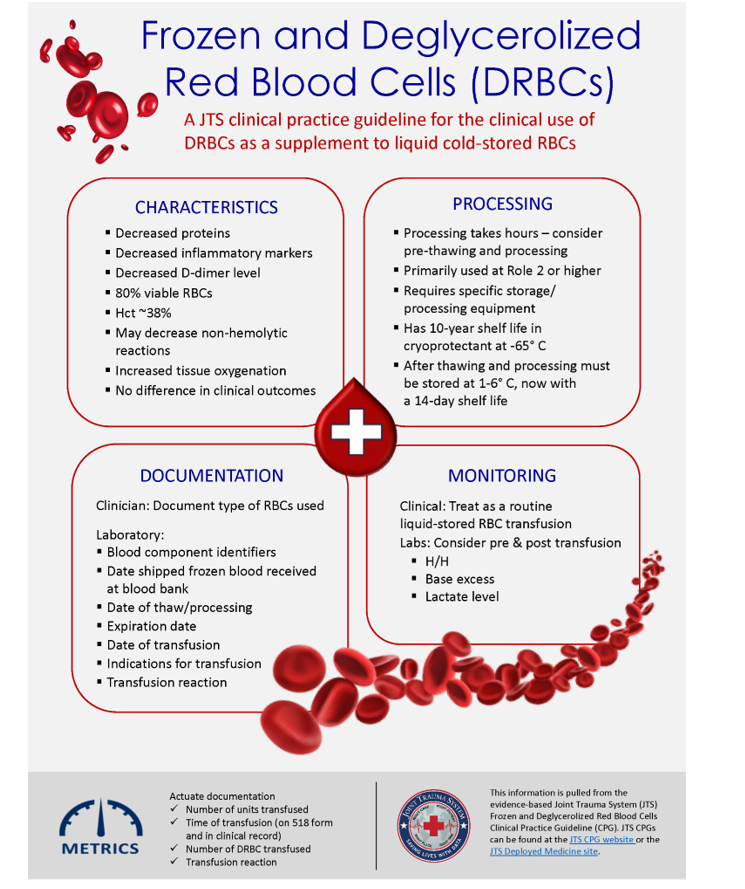
BACKGROUND
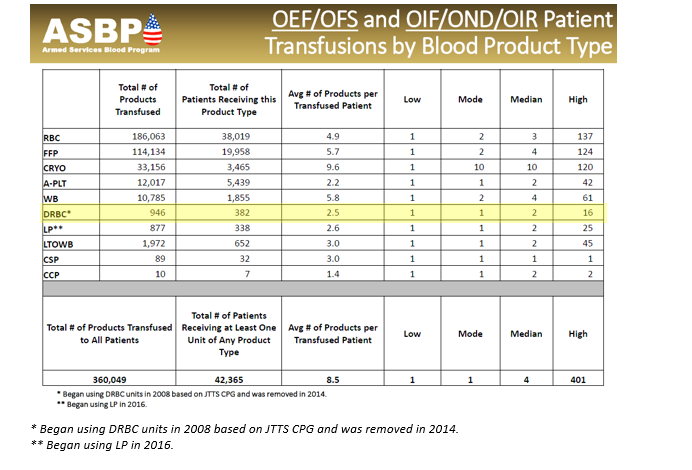
Frozen red blood cells are important in the military trauma system to maintain blood reserves in challenging operational environments. There are many clinical and operational limitations to using frozen red cells, but they remain an essential capability. However, whole blood is always preferable to using deglycerolized Red Blood Cells (DRBC). The idea behind a frozen blood (DRBC) reserve is twofold in the civilian system: 1.) to freeze units of rare blood types for later use by patients with special transfusion needs and 2.) managing special transfusion circumstances. For the military, the Navy is the primary user of DRBC to meet the needs of isolated maritime operational units with a Food and Drug Administration (FDA) licensed product, provide a licensed product when no other option exists, and for contingency reserves. Glycerol is the additive that is used to protect the RBCs from the effects of freezing. When the blood is thawed, the glycerol is removed, and the product is referred to as DRBC. The introduction of an automated, functionally closed system for glycerolization and deglycerolization of RBCs improved the operational practice and decreases the risk of bacterial contamination of the blood. The first operational frozen blood bank was established in 1956 at Chelsea Naval Hospital (Boston), in part to determine the practicality of frozen blood usage aboard Navy ships. In 1966, under Department of Defense (DoD) direction, the Navy Bureau of Medicine and Surgery established the first frozen blood bank in a combat zone at Naval Station Hospital, DaNang, Republic of South Vietnam. Over a 7-month period, 465 DRBC units were transfused to severely injured casualties. In the 1980s, the DoD froze 68,000 RBC units. Those units were pre-positioned throughout several geographic Combatant Commands in direct support of current and future military medical contingency operations. As of October 2023, just under 950 units of DRBC have been transfused to under 400 casualties. This compares to over 360,000 units of other blood products. (Table 1.) While DRBC have a role in transfusion therapy, they are less commonly used than other blood products and from an operational standpoint, have more of a use on hospital ships and amphibious warships in denied maritime environments. Navy hospital ships (USNS Comfort and USNS Mercy) and amphibious war ships routinely deploy with a standard amount frozen blood reserves roughly proportional to casualty holding capacity (Table 2). Aircraft carriers do not have DRBCs, although some have up to 5 units of stored whole blood generated from the ship’s crew. All the ships in table 2 have a routine walking blood bank (WBB) capability, but no stored platelets.
DRBCs are derived from 450 ml of whole blood collected in Citrate/Phosphate/Dextrose (CPD) or Citrate/Phosphate/Dextrose/Adenine (CPDA-1) collection bags. DRBCs can also be made from additive solution Red Blood Cell (RBC) units such as CPD/AS-1, CPD/AS-5 or CP2D/AS-3 if these are first centrifuged to concentrate the RBCs to hematocrit of about 75%. The RBCs are stored for up to 6 days at 1 – 6 °C before being frozen in a cryoprotectant (40% weight/volume glycerol) and stored in the frozen state at minus 65 °C or colder, for up to 10 years. 1
Once it is determined that they will be needed for transfusion, the frozen RBCs are thawed. They are then deglycerolized by sequential washing with hypertonic (12%) saline followed by normal (0.9%) saline mixed with 0.2% glucose. Following deglycerolization, units are re-suspended in AS-3 additive solution and stored at 1-6°C, until ready for transfusion. DRBCs must recover at least 80% of the RBCs present from the original unit. Units suspended in AS-3 are FDA-approved for transfusion for up to 14 days when processed on the Haemonetics Automated Cell Processor ACP215 (Figure 1), an FDA 510(k)-cleared, closed processing system device. DRBCs are licensed under the respective Service Blood Program licenses.1, 2 All U.S. hospital ships and amphibious warships (LHA, LHD, LPD) with DRBC have ACP215.
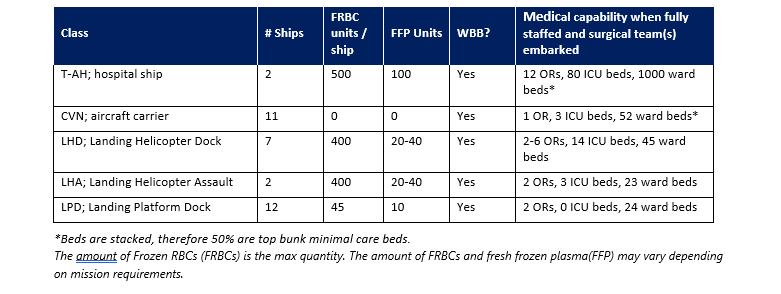
FRBC storage and DRBC processing are organic to select naval vessels (Table 2), some garrison medical treatment facilities (MTFs), and Service blood depots in the European Command, Africa Command, and Indo-Pacific Command areas of responsibility. Deployed MTFs and Medical Detachment Blood Support units do not have the organic mission or capability to store and process frozen RBCs. Use of this capability is based on operational necessity. Upon completion of the deglycerolization process, the produced DRBCs are typically provided to Role 2 and higher MTFs due to the 14-day shelf life.
Clinical Indications for Use
Each unit of DRBCs:
- Should be considered equivalent to a fresh unit of RBCs since they are frozen within 6 days of collection, washed during processing, and have a 14-day shelf-life upon deglycerolization.1
- Contains at least 80% of the RBCs present in the original unit of blood.
- Provides the same physiologic benefits as liquid RBCs.
- Carries the same expectation for post-transfusion survival as liquid-stored RBCs.
- Contains significantly lower concentrations of proteins associated with non-hemolytic transfusion reactions.
- The primary indication for use of frozen and deglycerolized RBCs is as a supplement to LTOWB, fresh whole blood and liquid RBCs during surge periods of increased transfusion requirements in order to decrease hemorrhagic morbidity and mortality.
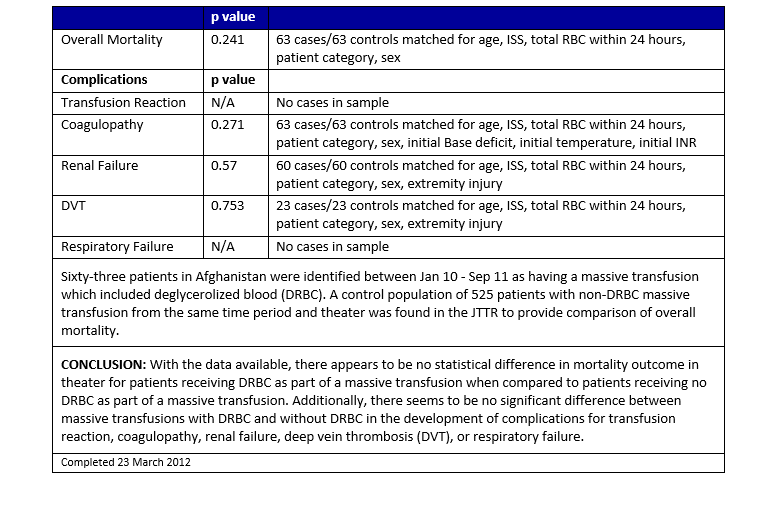
DRBCs may be used in lieu of liquid-stored RBCs for all RBC transfusion requirements including massive transfusions. The Joint Trauma System (JTS) Performance Improvement Branch analyzed data from the DoD Trauma Registry (DoDTR) and Massive Transfusion database and found no statistically significant difference in outcomes or transfusion-related complications between patients who received at least 1 unit of DRBCs as part of their massive transfusion and those who received only liquid-stored RBCs (See Appendix A). A single center prospective randomized trial comparing DRBCs to liquid-stored RBCs in stable trauma patients revealed decreased cytokines and D-dimer levels and increased tissue oxygenation measured by near infrared spectroscopy in patients transfused DRBCs. There were no differences in clinical outcomes. 2,4
- Transfusion monitoring:
- Clinical: Treat as a routine liquid-stored RBC transfusion before, during, and after transfusion, and for a suspected or actual adverse event.
- Laboratory: Consider obtaining pre- and post-transfusion Hemoglobin/Hematocrit and Base Excess/Deficit or lactate levels.
Documentation
Clinical documentation for a frozen and deglycerolized transfusion is the same as for a liquid transfusion. In addition:
- The physician may order use of DRBCs (for example, when washed RBCs would be preferred to reduce risk of transfusion reaction to plasma proteins in some patients), but in practice, the MTF will use DRBCs and liquid-stored RBCs interchangeably based on inventories and logistical considerations.
- The laboratory will establish and maintain a process to document DRBC transfusions in a manner that will facilitate future evaluation of recipients, including but not limited to:
- Blood component identifiers.
- Date of blood component receipt in frozen state.
- Date of thaw/deglycerolization/additive process and resulting expiration date.
- Casualty identifiers (including nationality and ABO/Rh categorization).
- Date of transfusion.
- Transfusion indication.
- Transfusion reaction, nature, and outcome.
Operational Considerations
Thawing and deglycerolization are time-consuming processes that will keep a well-staffed blood bank completely consumed. Thawing a frozen RBC unit takes about 35 minutes in a plasma thawer and about 45 minutes in a 42°C water bath. The process of deglycerolization of one blood unit on an Automated Cell Processor (ACP)-215 takes approximately one hour, not including time for initial unit thawing and labeling/release of deglycerolized product. When using multiple ACP215s, maximal throughput from initial thawing to unit release/availability can average up to 1 U every 2-3 hours per device. A laboratory technician may be trained to operate up to four ACP-215s at once. However, problems with device performance, supply limitations, breakage of the frozen RBC’s bag during thawing, and technician competency can lead to slower availability times. In periods of predictable operational requirements, it may be advisable to pre-thaw and deglycerolize several units so as not to incur the delay of preparation at the time they are needed.1

Performance Improvement (PI) Monitoring
INTENT (EXPECTED OUTCOMES)
All patients who receive DRBC transfusions have accurate documentation in the medical record of the quantity of transfused blood and any transfusion-related adverse events.
PERFORMANCE/ADHERENCE MEASURES
- In patients who were transfused DRBCs, there was accurate documentation, including time of transfusion and all transfused blood products annotated on the blood transfusion form (Appendix B) in the medical record and any transfusion-related adverse events.
- Accurate Documentation:
- Document number of units transfused
- Document time of transfusion (on 518 form and in clinical record)
- Document number of DRBC transfused
- Document transfusion reaction
DATA SOURCE
- Patient Record
- DoD Trauma Registry
- Combatant Command blood bank logs
- Theater Medical Data Store or its successor
The above constitutes the minimum criteria for PI monitoring of this CPG. System reporting will be performed twice a year; additional PI monitoring and system reporting may be performed as needed.
The system review and data analysis will be performed by the JTS Chief and the JTS PI team.
It is the combined responsibility of the trauma team leader and blood bank officer to ensure familiarity, appropriate compliance, and PI monitoring at the local level with this CPG.
References
- Valeri CR. Glycerolization and deglycerolization of red blood cells in a closed system using the Haemonetics ACP215, Naval Blood Research Laboratory
- Hampton DA, Wiles C, Fabricant LJ, Kiraly L, Differding J, Underwood S, Le D, Watters J, Schreiber MA. Cryopreserved red blood cells are superior to standard liquid red blood cells. J Trauma Acute Care Surg. 2014 Jul;77(1):20-7; discussion 26-7. PMID:24977750
- Emergency War Surgery Handbook, 5th Edition, 2018. Borden Institute, US Army Medical Department and School, Ft. Sam Houston, Texas.
- Fabricant L, Kiraly L, Wiles C, Differding J, Underwood S, Deloughery T, Schreiber M. Cryopreserved deglycerolized blood is safe and achieves superior tissue oxygenation compared with refrigerated red blood cells: a prospective randomized pilot study. J Trauma Acute Care Surg. 2013 Feb;74(2):371-6.
Appendix A: Massive Transfusion with DRBC Compared to Standard Massive Transfusion
Appendix B: 518 Blood or Blood Component Transfusion Record


Appendix C: DOTMLPF-P Considerations
The process of deglycerolizing requires laboratory technician training on the ACP215. This training may be conducted at unit level or as part of a formal course. Without training, a laboratory technician will not be able to operate an ACP215 and complete the procedure.
Deglycerolization requires robust medical logistics support for consumable supplies and ancillary equipment. The required supplies include (but are not limited to): AS-3, 12% sodium chloride solution, 0.9% Sodium Chloride-0.2% dextrose solution, and the ACP215 disposable sets.

Appendix D: Class VIII Medical Materiel
These materials are essential for the collection, storage, processing, and transfusion of frozen and deglycerolized red blood cells.
1. Whole blood collected in Citrate/Phosphate/Dextrose (CPD) or Citrate/Phosphate/Dextrose/Adenine (CPDA-1) collection bags
2. Additive solution Red Blood Cell (RBC) units such as CPD/AS-1, CPD/AS-5, or CP2D/AS-3
3. Cryoprotectant (40% w/v glycerol)
4. Hypertonic (12%) saline
5. Normal (0.9%) saline mixed with 0.2% glucose
6. AS-3 additive solution
7. Haemonetics Automated Cell Processor ACP215
8. Blood component identifiers
9. Plasma thawer or 37°C water bath ** must be validated for FRBC thawing **
10. Labels for documentation and release of deglycerolized product
11. Blood administration sets
12. Blood transfusion bags
13. Blood tubing with appropriate connectors
14. Intravenous catheters for transfusion administration
15. Infusion pumps or gravity infusion sets
16. Sterile gauze and tape for securing IV catheters
17. Blood pressure cuff and monitoring equipment
18. Anticoagulant solution for priming IV lines if necessary
19. IV pole for hanging blood transfusion bags
20. Personal protective equipment (gloves, gowns, masks) for healthcare providers involved in the transfusion process.
For additional information including National Stock Number (NSN), please contact dha.ncr.med-log.list.lpr-cps@health.mil
DISCLAIMER: This is not an exhaustive list. These are items identified to be important for the care of combat casualties.
Appendix E: Telemedicine/Teleconsultation
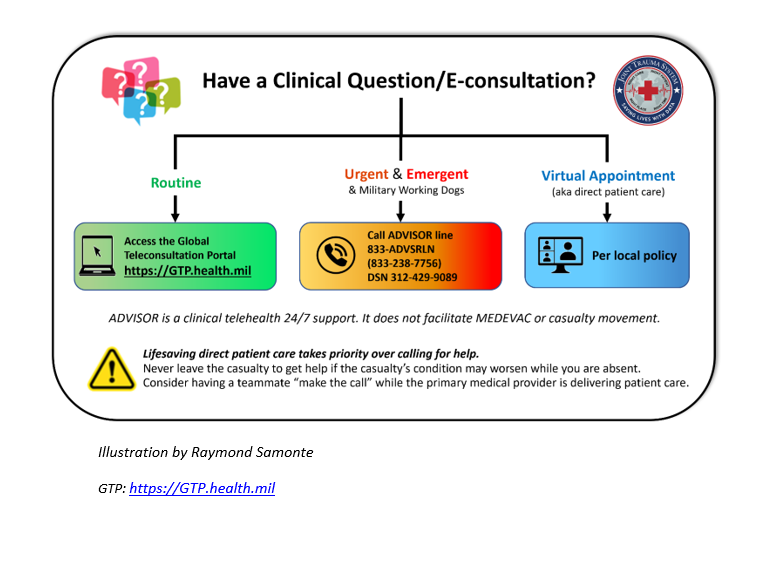
Appendix F: Information Regrading Off-Label Uses in CPGs
PURPOSE
The purpose of this Appendix is to ensure an understanding of DoD policy and practice regarding inclusion in CPGs of “off-label” uses of U.S. Food and Drug Administration (FDA)–approved products. This applies to off-label uses with patients who are armed forces members.
BACKGROUND
Unapproved (i.e. “off-label”) uses of FDA-approved products are extremely common in American medicine and are usually not subject to any special regulations. However, under Federal law, in some circumstances, unapproved uses of approved drugs are subject to FDA regulations governing “investigational new drugs.” These circumstances include such uses as part of clinical trials, and in the military context, command required, unapproved uses. Some command requested unapproved uses may also be subject to special regulations.
ADDITIONAL INFORMATION REGARDING OFF-LABEL USES IN CPGS
The inclusion in CPGs of off-label uses is not a clinical trial, nor is it a command request or requirement. Further, it does not imply that the Military Health System requires that use by DoD health care practitioners or considers it to be the “standard of care.” Rather, the inclusion in CPGs of off-label uses is to inform the clinical judgment of the responsible health care practitioner by providing information regarding potential risks and benefits of treatment alternatives. The decision is for the clinical judgment of the responsible health care practitioner within the practitioner-patient relationship.
ADDITIONAL PROCEDURES
Balanced Discussion
Consistent with this purpose, CPG discussions of off-label uses specifically state that they are uses not approved by the FDA. Further, such discussions are balanced in the presentation of appropriate clinical study data, including any such data that suggest caution in the use of the product and specifically including any FDA-issued warnings.
Quality Assurance Monitoring
With respect to such off-label uses, DoD procedure is to maintain a regular system of quality assurance monitoring of outcomes and known potential adverse events. For this reason, the importance of accurate clinical records is underscored.
Information to Patients
Good clinical practice includes the provision of appropriate information to patients. Each CPG discussing an unusual off-label use will address the issue of information to patients. When practicable, consideration will be given to including in an appendix an appropriate information sheet for distribution to patients, whether before or after use of the product. Information to patients should address in plain language: a) that the use is not approved by the FDA; b) the reasons why a DoD health care practitioner would decide to use the product for this purpose; and c) the potential risks associated with such use.





